STING-Triggered CNS Inflammation in Human Neurodegenerative Diseases
- PMID: 37239045
- PMCID: PMC10216406
- DOI: 10.3390/biomedicines11051375
STING-Triggered CNS Inflammation in Human Neurodegenerative Diseases
Abstract
Background: Some neurodegenerative diseases have an element of neuroinflammation that is triggered by viral nucleic acids, resulting in the generation of type I interferons. In the cGAS-STING pathway, microbial and host-derived DNA bind and activate the DNA sensor cGAS, and the resulting cyclic dinucleotide, 2'3-cGAMP, binds to a critical adaptor protein, stimulator of interferon genes (STING), which leads to activation of downstream pathway components. However, there is limited work demonstrating the activation of the cGAS-STING pathway in human neurodegenerative diseases.
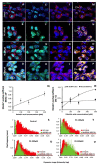
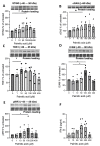
Methods: Post-mortem CNS tissue from donors with multiple sclerosis (n = 4), Alzheimer's disease (n = 6), Parkinson's disease (n = 3), amyotrophic lateral sclerosis (n = 3) and non-neurodegenerative controls (n = 11) were screened by immunohistochemistry for STING and relevant protein aggregates (e.g., amyloid-β, α-synuclein, TDP-43). Human brain endothelial cells were cultured and stimulated with the STING agonist palmitic acid (1-400 μM) and assessed for mitochondrial stress (release of mitochondrial DNA into cytosol, increased oxygen consumption), downstream regulator factors, TBK-1/pIRF3 and inflammatory biomarker interferon-β release and changes in ICAM-1 integrin expression.
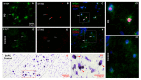
Results: In neurodegenerative brain diseases, elevated STING protein was observed mainly in brain endothelial cells and neurons, compared to non-neurodegenerative control tissues where STING protein staining was weaker. Interestingly, a higher STING presence was associated with toxic protein aggregates (e.g., in neurons). Similarly high STING protein levels were observed within acute demyelinating lesions in multiple sclerosis subjects. To understand non-microbial/metabolic stress activation of the cGAS-STING pathway, brain endothelial cells were treated with palmitic acid. This evoked mitochondrial respiratory stress up to a ~2.5-fold increase in cellular oxygen consumption. Palmitic acid induced a statistically significant increase in cytosolic DNA leakage from endothelial cell mitochondria (Mander's coefficient; p < 0.05) and a significant increase in TBK-1, phosphorylated transcription factor IFN regulatory factor 3, cGAS and cell surface ICAM. In addition, a dose response in the secretion of interferon-β was observed, but it failed to reach statistical significance.
Conclusions: The histological evidence shows that the common cGAS-STING pathway appears to be activated in endothelial and neural cells in all four neurodegenerative diseases examined. Together with the in vitro data, this suggests that the STING pathway might be activated via perturbation of mitochondrial stress and DNA leakage, resulting in downstream neuroinflammation; hence, this pathway may be a target for future STING therapeutics.
Keywords: cGAS; cerebral endothelial cells; cortical neurons; neuroinflammation; palmitic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Reinert L.S., Lopusna K., Winther H., Sun C., Thomsen M.K., Nandakumar R., Mogensen T.H., Meyer M., Vaegter C., Nyengaard J.R., et al. Sensing of HSV-1 by the cGAS-STING pathway in microglia orchestrates antiviral defence in the CNS. Nat. Commun. 2016;7:13348. doi: 10.1038/ncomms13348. - DOI - PMC - PubMed
-
- Gutowski N.J., Ferecsko A.S., Smallwood M., Holley J., Newcombe J., Moore A., Eggleton P. Human neurodegenerative CNS tissues show robust expression of cGAS-STING pathway components in association with inflammation. Eur. J. Neurol. 2019;26:717.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

