Combination L-Glutamine with Gemcitabine and Nab-Paclitaxel in Treatment-Naïve Advanced Pancreatic Cancer: The Phase I GlutaPanc Study Protocol
- PMID: 37239063
- PMCID: PMC10216251
- DOI: 10.3390/biomedicines11051392
Combination L-Glutamine with Gemcitabine and Nab-Paclitaxel in Treatment-Naïve Advanced Pancreatic Cancer: The Phase I GlutaPanc Study Protocol
Abstract
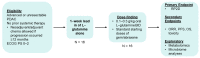
Advanced pancreatic cancer is underscored by progressive therapeutic resistance and a dismal 5-year survival rate of 3%. Preclinical data demonstrated glutamine supplementation, not deprivation, elicited antitumor effects against pancreatic ductal adenocarcinoma (PDAC) alone and in combination with gemcitabine in a dose-dependent manner. The GlutaPanc phase I trial is a single-arm, open-label clinical trial investigating the safety of combination L-glutamine, gemcitabine, and nab-paclitaxel in subjects (n = 16) with untreated, locally advanced unresectable or metastatic pancreatic cancer. Following a 7-day lead-in phase with L-glutamine, the dose-finding phase via Bayesian design begins with treatment cycles lasting 28 days until disease progression, intolerance, or withdrawal. The primary objective is to establish the recommended phase II dose (RP2D) of combination L-glutamine, gemcitabine, and nab-paclitaxel. Secondary objectives include safety of the combination across all dose levels and preliminary evidence of antitumor activity. Exploratory objectives include evaluating changes in plasma metabolites across multiple time points and changes in the stool microbiome pre and post L-glutamine supplementation. If this phase I clinical trial demonstrates the feasibility of L-glutamine in combination with nab-paclitaxel and gemcitabine, we would advance the development of this combination as a first-line systemic option in subjects with metastatic pancreatic cancer, a high-risk subgroup desperately in need of additional therapies.
Keywords: L-glutamine; chemotherapy; clinical trial; gemcitabine; metastatic; nab-paclitaxel; pancreatic cancer.
Conflict of interest statement
Authors J.G., A.E.H., H.M. and N.B. are co-inventors of international patent application number PCT/US2021/043961, filed 30 July 2021.
Figures
References
-
- Aprile G., Negri F.V., Giuliani F., De Carlo E., Melisi D., Simionato F., Silvestris N., Brunetti O., Leone F., Marino D., et al. Second-line chemotherapy for advanced pancreatic cancer: Which is the best option? Crit. Rev. Oncol. Hematol. 2017;115:1–12. doi: 10.1016/j.critrevonc.2017.03.025. - DOI - PubMed

