An Insight into Cellular and Molecular Mechanisms Underlying the Pathogenesis of Neurodegeneration in Alzheimer's Disease
- PMID: 37239068
- PMCID: PMC10216658
- DOI: 10.3390/biomedicines11051398
An Insight into Cellular and Molecular Mechanisms Underlying the Pathogenesis of Neurodegeneration in Alzheimer's Disease
Abstract
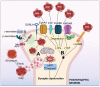
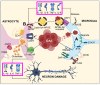
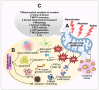
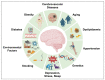
Alzheimer's disease (AD) is the most prominent neurodegenerative disorder in the aging population. It is characterized by cognitive decline, gradual neurodegeneration, and the development of amyloid-β (Aβ)-plaques and neurofibrillary tangles, which constitute hyperphosphorylated tau. The early stages of neurodegeneration in AD include the loss of neurons, followed by synaptic impairment. Since the discovery of AD, substantial factual research has surfaced that outlines the disease's causes, molecular mechanisms, and prospective therapeutics, but a successful cure for the disease has not yet been discovered. This may be attributed to the complicated pathogenesis of AD, the absence of a well-defined molecular mechanism, and the constrained diagnostic resources and treatment options. To address the aforementioned challenges, extensive disease modeling is essential to fully comprehend the underlying mechanisms of AD, making it easier to design and develop effective treatment strategies. Emerging evidence over the past few decades supports the critical role of Aβ and tau in AD pathogenesis and the participation of glial cells in different molecular and cellular pathways. This review extensively discusses the current understanding concerning Aβ- and tau-associated molecular mechanisms and glial dysfunction in AD. Moreover, the critical risk factors associated with AD including genetics, aging, environmental variables, lifestyle habits, medical conditions, viral/bacterial infections, and psychiatric factors have been summarized. The present study will entice researchers to more thoroughly comprehend and explore the current status of the molecular mechanism of AD, which may assist in AD drug development in the forthcoming era.
Keywords: Alzheimer’s disease; amyloid plaques; molecular mechanism; risk factors; tau tangles.
Conflict of interest statement
The authors declare no conflicts of interest concerning this article’s research, authorship, and publication.
Figures


References
-
- de Sá-Caputo D.D.C., Mario Bernardo-Filho A.S., Taiar R. Introductory Chapter: Neurological Disorders-Therapy Approaches. IntechOpen; London, UK: 2021. pp. 1–11.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

