Collagens Regulating Adipose Tissue Formation and Functions
- PMID: 37239083
- PMCID: PMC10216475
- DOI: 10.3390/biomedicines11051412
Collagens Regulating Adipose Tissue Formation and Functions
Abstract
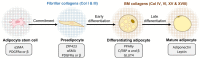
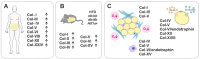
The globally increasing prevalence of obesity is associated with the development of metabolic diseases such as type 2 diabetes, dyslipidemia, and fatty liver. Excess adipose tissue (AT) often leads to its malfunction and to a systemic metabolic dysfunction because, in addition to storing lipids, AT is an active endocrine system. Adipocytes are embedded in a unique extracellular matrix (ECM), which provides structural support to the cells as well as participating in the regulation of their functions, such as proliferation and differentiation. Adipocytes have a thin pericellular layer of a specialized ECM, referred to as the basement membrane (BM), which is an important functional unit that lies between cells and tissue stroma. Collagens form a major group of proteins in the ECM, and some of them, especially the BM-associated collagens, support AT functions and participate in the regulation of adipocyte differentiation. In pathological conditions such as obesity, AT often proceeds to fibrosis, characterized by the accumulation of large collagen bundles, which disturbs the natural functions of the AT. In this review, we summarize the current knowledge on the vertebrate collagens that are important for AT development and function and include basic information on some other important ECM components, principally fibronectin, of the AT. We also briefly discuss the function of AT collagens in certain metabolic diseases in which they have been shown to play central roles.
Keywords: adipogenesis; basement membrane; collagen; diabetes; dyslipidemia; extracellular matrix; fibronectin; fibrosis; lipodystrophy; obesity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

