Checkpoint Inhibitor-Induced Colitis: An Update
- PMID: 37239166
- PMCID: PMC10216810
- DOI: 10.3390/biomedicines11051496
Checkpoint Inhibitor-Induced Colitis: An Update
Abstract
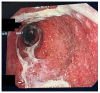
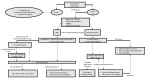
Immunotherapy with immune checkpoint inhibitors (ICIs) nowadays has indications for several solid tumors. The current targets for ICIs are CTLA-4, PD-1, and PD-L1 receptors. Despite the clinical advantages derived from ICIs, a variety of side effects are linked to overstimulation of the immune system. Among these, ICI-related colitis is one of the most common, with a disabling impact on the patient. Diarrhea, abdominal pain, abdominal distension, cramping, and hematochezia are the most common ICI enterocolitis presenting symptoms. The most frequently used grading system for assessment of the severity of ICI enterocolitis is called the Common Terminology Criteria for Adverse Events (CTCAE) grading. With regard to the histological picture, there is no specific feature; however, microscopic damage can be classified into five types: (1) acute active colitis, (2) chronic active colitis, (3) microscopic colitis-like, (4) graft-versus-host disease-like, and (5) other types. Supportive therapy (oral hydration, a bland diet without lactose or caffeine, and anti-diarrheal agents) is indicated in mild colitis. Symptomatic treatment alone or with loperamide, a low-fiber diet, and spasmolytics are recommended for low-grade diarrhea. In more severe cases, corticosteroid treatment is mandatory. In refractory cases, off-label use of biological therapies (infliximab or vedolizumab) was proposed.
Keywords: cancer; colitis; diarrhea; immune checkpoint inhibitors; immunotherapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Paz-Ares L., Ciuleanu T.-E., Cobo M., Schenker M., Zurawski B., Menezes J., Richardet E., Bennouna J., Felip E., Juan-Vidal O., et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): An international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:198–211. doi: 10.1016/S1470-2045(20)30641-0. Correction in Lancet Oncol. 2021, 22, e92. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

