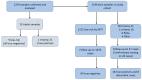
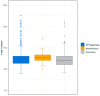
Clinical Experience with Genome-Wide Noninvasive Prenatal Screening in a Large Cohort of Twin Pregnancies
- PMID: 37239342
- PMCID: PMC10218036
- DOI: 10.3390/genes14050982
Clinical Experience with Genome-Wide Noninvasive Prenatal Screening in a Large Cohort of Twin Pregnancies
Abstract
Non-invasive prenatal screening (NIPS) in twin gestations has been shown to have high detection rates and low false-positive rates for trisomy 21, as seen in singleton pregnancies, although there have been few large cohort twin studies, genome-wide studies in particular, to date. In this study, we looked at the performance of genome-wide NIPT in a large cohort consisting of 1244 twin pregnancy samples collected over a two-year period in a single laboratory in Italy. All samples underwent an NIPS for common trisomies, with 61.5% of study participants choosing to undergo genome-wide NIPS for additional fetal anomalies (namely, rare autosomal aneuploidies and CNVs). There were nine initial no-call results, all of which were resolved upon retest. Based on our NIPS results, 17 samples were at high risk for trisomy 21, one for trisomy 18, six for a rare autosomal aneuploidy, and four for a CNV. Clinical follow-up was available for 27 out of 29 high-risk cases; a sensitivity of 100%, a specificity of 99.9%, and a PPV of 94.4% were noted for trisomy 21. Clinical follow-up was also available for 1110 (96.6%) of the low-risk cases, all of which were true negatives. In conclusion, we found that NIPS was a reliable screening approach for trisomy 21 in twin pregnancies.
Keywords: failure rate; genome-wide sequencing; non-invasive prenatal testing; sensitivity; specificity; twin pregnancies.
Conflict of interest statement
Authors L.D.F., G.S., T.S., P.S., N.P., M.I., R.R., A.M. and C.R. are employed by AMES. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Palomaki G.E., Chiu R.W.K., Pertile M.D., Sistermans E.A., Yaron Y., Vermeesch J.R., Vora N.L., Best R.G., Wilkins-Haug L. International Society for Prenatal Diagnosis Position Statement: Cell free (cf) DNA screening for Down syndrome in multiple pregnancies. Prenat. Diagn. 2020;41:1222–1232. doi: 10.1002/pd.5832. - DOI - PubMed
-
- Pison G., Monden C., Smits J. Twinning Rates in Developed Countries: Trends and Explanations. Popul. Dev. Rev. 2015;41:629–649. doi: 10.1111/j.1728-4457.2015.00088.x. - DOI
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous