Potential Clinically Relevant Effects of Sialylation on Human Serum AAG-Drug Interactions Assessed by Isothermal Titration Calorimetry: Insight into Pharmacoglycomics?
- PMID: 37239819
- PMCID: PMC10218007
- DOI: 10.3390/ijms24108472
Potential Clinically Relevant Effects of Sialylation on Human Serum AAG-Drug Interactions Assessed by Isothermal Titration Calorimetry: Insight into Pharmacoglycomics?
Abstract
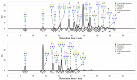
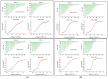
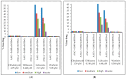
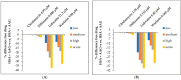
Human serum alpha-1 acid glycoprotein is an acute-phase plasma protein involved in the binding and transport of many drugs, especially basic and lipophilic substances. It has been reported that the sialic acid groups that terminate the N-glycan chains of alpha-1 acid glycoprotein change in response to certain health conditions and may have a major impact on drug binding to alpha-1 acid glycoprotein. The interaction between native or desialylated alpha-1 acid glycoprotein and four representative drugs-clindamycin, diltiazem, lidocaine, and warfarin-was quantitatively evaluated using isothermal titration calorimetry. The calorimetry assay used here is a convenient and widely used approach to directly measure the amount of heat released or absorbed during the association processes of biomolecules in solution and to quantitatively estimate the thermodynamics of the interaction. The results showed that the binding of drugs with alpha-1 acid glycoprotein were enthalpy-driven exothermic interactions, and the binding affinity was in the range of 10-5-10-6 M. Desialylated alpha-1 acid glycoprotein showed significantly different binding with diltiazem, lidocaine, and warfarin compared with native alpha-1 acid glycoprotein, whereas clindamycin showed no significant difference. Therefore, a different degree of sialylation may result in different binding affinities, and the clinical significance of changes in sialylation or glycosylation of alpha-1 acid glycoprotein in general should not be neglected.
Keywords: alpha-1 acid glycoprotein; binding affinity; drugs; isothermal titration calorimetry; plasma protein binding; sialic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Kragh-Hansen U. Molecular aspects of ligand binding to serum albumin. Pharmacol. Rev. 1981;33:17–53. - PubMed
-
- Mehvar R. Role of Protein Binding in Pharmacokinetics. Am. J. Pharm. Educ. 2005;69:1526. doi: 10.5688/aj69051526. - DOI
-
- Kremer J.M., Wilting J., Janssen L.H. Drug binding to human alpha-1-acid glycoprotein in health and disease. Pharmacol. Rev. 1988;40:1–47. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

