Supplementation with a New Standardized Extract of Green and Black Tea Exerts Antiadipogenic Effects and Prevents Insulin Resistance in Mice with Metabolic Syndrome
- PMID: 37239868
- PMCID: PMC10218622
- DOI: 10.3390/ijms24108521
Supplementation with a New Standardized Extract of Green and Black Tea Exerts Antiadipogenic Effects and Prevents Insulin Resistance in Mice with Metabolic Syndrome
Abstract
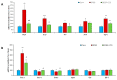
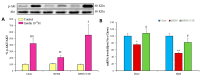
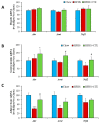
Insulin resistance is one of the main characteristics of metabolic syndrome (MetS) and the main cause of the development of type II diabetes. The high prevalence of this syndrome in recent decades has made it necessary to search for preventive and therapeutic agents, ideally of natural origin, with fewer side effects than conventional pharmacological treatments. Tea is widely known for its medicinal properties, including beneficial effects on weight management and insulin resistance. The aim of this study was to analyze whether a standardized extract of green and black tea (ADM® Complex Tea Extract (CTE)) prevents the development of insulin resistance in mice with MetS. For this purpose, C57BL6/J mice were fed for 20 weeks with a standard diet (Chow), a diet with 56% kcal from fat and sugar (HFHS) or an HFHS diet supplemented with 1.6% CTE. CTE supplementation reduced body weight gain, adiposity and circulating leptin levels. Likewise, CTE also exerted lipolytic and antiadipogenic effects in 3T3-L1 adipocyte cultures and in the C. elegans model. Regarding insulin resistance, CTE supplementation significantly increased plasma adiponectin concentrations and reduced the circulating levels of insulin and the HOMA-IR. Incubation of liver, gastrocnemius muscle and retroperitoneal adipose tissue explants with insulin increased the pAkt/Akt ratio in mice fed with Chow and HFHS + CTE but not in those fed only with HFHS. The greater activation of the PI3K/Akt pathway in response to insulin in mice supplemented with CTE was associated with a decrease in the expression of the proinflammatory markers Mcp-1, IL-6, IL-1β or Tnf-α and with an overexpression of the antioxidant enzymes Sod-1, Gpx-3, Ho-1 and Gsr in these tissues. Moreover, in skeletal muscle, mice treated with CTE showed increased mRNA levels of the aryl hydrocarbon receptor (Ahr), Arnt and Nrf2, suggesting that the CTE's insulin-sensitizing effects could be the result of the activation of this pathway. In conclusion, supplementation with the standardized extract of green and black tea CTE reduces body weight gain, exerts lipolytic and antiadipogenic effects and reduces insulin resistance in mice with MetS through its anti-inflammatory and antioxidant effects.
Keywords: antioxidant; black tea; extract; green tea; insulin resistance; metabolic syndrome; obesity.
Conflict of interest statement
Since this work was carried out in collaboration with the company ADM Wild, authors from this company may have conflict of interest. These authors have participated in the characterization and production of the extracts and in the studies of adipogenesis in the
Figures







References
-
- WHO. 2016. [(accessed on 27 March 2023)]. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

