Old Drug, New Delivery Strategy: MMAE Repackaged
- PMID: 37239890
- PMCID: PMC10218109
- DOI: 10.3390/ijms24108543
Old Drug, New Delivery Strategy: MMAE Repackaged
Abstract
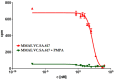
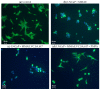
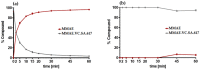
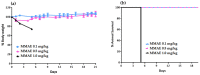
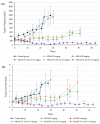
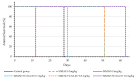
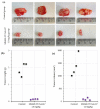
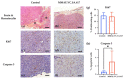
Targeting therapy is a concept that has gained significant importance in recent years, especially in oncology. The severe dose-limiting side effects of chemotherapy necessitate the development of novel, efficient and tolerable therapy approaches. In this regard, the prostate specific membrane antigene (PSMA) has been well established as a molecular target for diagnosis of, as well as therapy for, prostate cancer. Although most PSMA-targeting ligands are radiopharmaceuticals used in imaging or radioligand therapy, this article evaluates a PSMA-targeting small molecule-drug conjugate, and, thus, addresses a hitherto little-explored field. PSMA binding affinity and cytotoxicity were determined in vitro using cell-based assays. Enzyme-specific cleavage of the active drug was quantified via an enzyme-based assay. Efficacy and tolerability in vivo were assessed using an LNCaP xenograft model. Histopathological characterization of the tumor in terms of apoptotic status and proliferation rate was carried out using caspase-3 and Ki67 staining. The binding affinity of the Monomethyl auristatin E (MMAE) conjugate was moderate, compared to the drug-free PSMA ligand. Cytotoxicity in vitro was in the nanomolar range. Both binding and cytotoxicity were found to be PSMA-specific. Additionally, complete MMAE release could be reached after incubation with cathepsin B. In vivo, the MMAE conjugate displayed good tolerability and dose-dependent inhibition of tumor growth. Immunohistochemical and histological studies revealed the antitumor effect of MMAE.VC.SA.617, resulting in the inhibition of proliferation and the enhancement of apoptosis. The developed MMAE conjugate showed good properties in vitro, as well as in vivo, and should, therefore, be considered a promising candidate for a translational approach.
Keywords: MMAE; PSMA; drug targeting; prostate cancer; small molecule–drug conjugates; therapeutic efficacy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

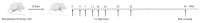

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

