The Role of Krüppel-like Factors in Pancreatic Physiology and Pathophysiology
- PMID: 37239940
- PMCID: PMC10218095
- DOI: 10.3390/ijms24108589
The Role of Krüppel-like Factors in Pancreatic Physiology and Pathophysiology
Abstract
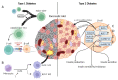
Krüppel-like factors (KLFs) belong to the family of transcription factors with three highly conserved zinc finger domains in the C-terminus. They regulate homeostasis, development, and disease progression in many tissues. It has been shown that KLFs play an essential role in the endocrine and exocrine compartments of the pancreas. They are necessary to maintain glucose homeostasis and have been implicated in the development of diabetes. Furthermore, they can be a vital tool in enabling pancreas regeneration and disease modeling. Finally, the KLF family contains proteins that act as tumor suppressors and oncogenes. A subset of members has a biphasic function, being upregulated in the early stages of oncogenesis and stimulating its progression and downregulated in the late stages to allow for tumor dissemination. Here, we describe KLFs' function in pancreatic physiology and pathophysiology.
Keywords: Krüppel-like factors; metabolism; pancreatic cancer; pancreatitis; reprograming; stemness.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

