Impact of Endurance Training on Regeneration of Axons, Glial Cells, and Inhibitory Neurons after Spinal Cord Injury: A Link between Functional Outcome and Regeneration Potential within the Lesion Site and in Adjacent Spinal Cord Tissue
- PMID: 37239968
- PMCID: PMC10218115
- DOI: 10.3390/ijms24108616
Impact of Endurance Training on Regeneration of Axons, Glial Cells, and Inhibitory Neurons after Spinal Cord Injury: A Link between Functional Outcome and Regeneration Potential within the Lesion Site and in Adjacent Spinal Cord Tissue
Abstract
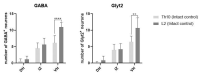
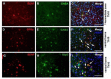
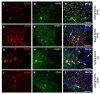
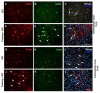
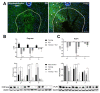
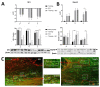
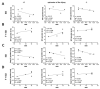
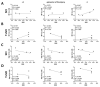
Endurance training prior to spinal cord injury (SCI) has a beneficial effect on the activation of signaling pathways responsible for survival, neuroplasticity, and neuroregeneration. It is, however, unclear which training-induced cell populations are essential for the functional outcome after SCI. Adult Wistar rats were divided into four groups: control, six weeks of endurance training, Th9 compression (40 g/15 min), and pretraining + Th9 compression. The animals survived six weeks. Training alone increased the gene expression and protein level of immature CNP-ase oligodendrocytes (~16%) at Th10, and caused rearrangements in neurotrophic regulation of inhibitory GABA/glycinergic neurons at the Th10 and L2 levels, known to contain the interneurons with rhythmogenic potential. Training + SCI upregulated markers for immature and mature (CNP-ase, PLP1) oligodendrocytes by ~13% at the lesion site and caudally, and increased the number of GABA/glycinergic neurons in specific spinal cord regions. In the pretrained SCI group, the functional outcome of hindlimbs positively correlated with the protein levels of CNP-ase, PLP1, and neurofilaments (NF-l), but not with the outgrowing axons (Gap-43) at the lesion site and caudally. These results indicate that endurance training applied before SCI potentiates the repair in damaged spinal cord, and creates a suitable environment for neurological outcome.
Keywords: endurance training; neurological outcome; regeneration of cell populations; spinal cord compression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Activation of Three Major Signaling Pathways After Endurance Training and Spinal Cord Injury.Mol Neurobiol. 2022 Feb;59(2):950-967. doi: 10.1007/s12035-021-02628-y. Epub 2021 Nov 22. Mol Neurobiol. 2022. PMID: 34811634 Free PMC article.
-
Expressing Constitutively Active Rheb in Adult Neurons after a Complete Spinal Cord Injury Enhances Axonal Regeneration beyond a Chondroitinase-Treated Glial Scar.J Neurosci. 2015 Aug 5;35(31):11068-80. doi: 10.1523/JNEUROSCI.0719-15.2015. J Neurosci. 2015. PMID: 26245968 Free PMC article.
-
EphA4 Obstructs Spinal Cord Neuron Regeneration by Promoting Excessive Activation of Astrocytes.Cell Mol Neurobiol. 2022 Jul;42(5):1557-1568. doi: 10.1007/s10571-021-01046-x. Epub 2021 Feb 17. Cell Mol Neurobiol. 2022. PMID: 33595805 Free PMC article.
-
The Biology of Regeneration Failure and Success After Spinal Cord Injury.Physiol Rev. 2018 Apr 1;98(2):881-917. doi: 10.1152/physrev.00017.2017. Physiol Rev. 2018. PMID: 29513146 Free PMC article. Review.
-
Molecular targets in spinal cord injury.J Mol Med (Berl). 2005 Sep;83(9):657-71. doi: 10.1007/s00109-005-0663-3. Epub 2005 Aug 2. J Mol Med (Berl). 2005. PMID: 16075258 Review.
Cited by
-
NeuroAiDTM-II (MLC901) Promoted Neurogenesis by Activating the PI3K/AKT/GSK-3β Signaling Pathway in Rat Spinal Cord Injury Models.Biomedicines. 2024 Aug 21;12(8):1920. doi: 10.3390/biomedicines12081920. Biomedicines. 2024. PMID: 39200383 Free PMC article.
-
Effect of high-intensity exercise training on functional recovery after spinal cord injury.Front Neurol. 2025 Feb 17;16:1442004. doi: 10.3389/fneur.2025.1442004. eCollection 2025. Front Neurol. 2025. PMID: 40035032 Free PMC article. Review.
-
The interplay between BDNF and PGC-1 alpha in maintaining brain health: role of exercise.Front Endocrinol (Lausanne). 2024 Aug 22;15:1433750. doi: 10.3389/fendo.2024.1433750. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39239097 Free PMC article. Review.
-
Body weight-supported treadmill training reduces glial scar overgrowth in SCI rats by decreasing the reactivity of astrocytes during the subacute phase.BMC Neurosci. 2025 Apr 28;26(1):30. doi: 10.1186/s12868-025-00947-7. BMC Neurosci. 2025. PMID: 40295901 Free PMC article.
-
Spinal Cord Injury Remyelination: Pathways to Therapies.Int J Mol Sci. 2025 Jul 26;26(15):7249. doi: 10.3390/ijms26157249. Int J Mol Sci. 2025. PMID: 40806380 Free PMC article. Review.
References
-
- Arrieta H., Rezola-Pardo C., Echeverria I., Iturburu M., Gil S.M., Yanguas J.J., Irazusta J., Rodriguez-Larrad A. Physical activity and fitness are associated with verbal memory, quality of life and depression among nursing home residents: Preliminary data of a randomized controlled trial. BMC Geriatr. 2018;18:80. doi: 10.1186/s12877-018-0770-y. - DOI - PMC - PubMed
-
- Schön M., Malenovská K.M., Nemec M., Laiferová N.A., Straka I., Košutzká Z., Matejička P., Valkovič P., Ukropec J., Ukropcová B. Acute endurance exercise modulates growth differentiation factor 11 in cerebrospinal fluid of healthy young adults. Front. Endocrinol. 2023;14:1137048. doi: 10.3389/fendo.2023.1137048. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- VEGA-2/0145/21 and VEGA-2/0098/20/Grant Agency of the Ministry of Education Science, Research and Sport of the Slovak Republic and the Slovak Academy of Sciences
- APVV-15-0766, APVV-19-0324 and APVV-18-0163/Slovak Research and Developmental Agency
- IMTS: 313011V344/Research & Development Operational Programme funded by the European Regional Development Fund
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

