Potential Role of the mTORC1-PGC1α-PPARα Axis under Type-II Diabetes and Hypertension in the Human Heart
- PMID: 37239977
- PMCID: PMC10218005
- DOI: 10.3390/ijms24108629
Potential Role of the mTORC1-PGC1α-PPARα Axis under Type-II Diabetes and Hypertension in the Human Heart
Abstract
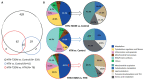
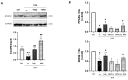
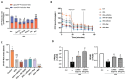
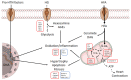
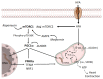
Type-2 diabetes (T2DM) and arterial hypertension (HTN) are major risk factors for heart failure. Importantly, these pathologies could induce synergetic alterations in the heart, and the discovery of key common molecular signaling may suggest new targets for therapy. Intraoperative cardiac biopsies were obtained from patients with coronary heart disease and preserved systolic function, with or without HTN and/or T2DM, who underwent coronary artery bypass grafting (CABG). Control (n = 5), HTN (n = 7), and HTN + T2DM (n = 7) samples were analysed by proteomics and bioinformatics. Additionally, cultured rat cardiomyocytes were used for the analysis (protein level and activation, mRNA expression, and bioenergetic performance) of key molecular mediators under stimulation of main components of HTN and T2DM (high glucose and/or fatty acids and angiotensin-II). As results, in cardiac biopsies, we found significant alterations of 677 proteins and after filtering for non-cardiac factors, 529 and 41 were changed in HTN-T2DM and in HTN subjects, respectively, against the control. Interestingly, 81% of proteins in HTN-T2DM were distinct from HTN, while 95% from HTN were common with HTN-T2DM. In addition, 78 factors were differentially expressed in HTN-T2DM against HTN, predominantly downregulated proteins of mitochondrial respiration and lipid oxidation. Bioinformatic analyses suggested the implication of mTOR signaling and reduction of AMPK and PPARα activation, and regulation of PGC1α, fatty acid oxidation, and oxidative phosphorylation. In cultured cardiomyocytes, an excess of the palmitate activated mTORC1 complex and subsequent attenuation of PGC1α-PPARα transcription of β-oxidation and mitochondrial electron chain factors affect mitochondrial/glycolytic ATP synthesis. Silencing of PGC1α further reduced total ATP and both mitochondrial and glycolytic ATP. Thus, the coexistence of HTN and T2DM induced higher alterations in cardiac proteins than HTN. HTN-T2DM subjects exhibited a marked downregulation of mitochondrial respiration and lipid metabolism and the mTORC1-PGC1α-PPARα axis might account as a target for therapeutical strategies.
Keywords: cardiomyopathy; hypertension; mTOR complexes; type-II diabetes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Yap J., Tay W.T., Teng T.K., Anand I., Richards A.M., Ling L.H., MacDonald M.R., Chandramouli C., Tromp J., Siswanto B.B., et al. Association of Diabetes Mellitus on Cardiac Remodeling, Quality of Life, and Clinical Outcomes in Heart Failure With Reduced and Preserved Ejection Fraction. J. Am. Heart Assoc. 2019;8:e013114. doi: 10.1161/JAHA.119.013114. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

