Boosting the Antibacterial Activity of Azithromycin on Multidrug-Resistant Escherichia coli by Efflux Pump Inhibition Coupled with Outer Membrane Permeabilization Induced by Phenylalanine-Arginine β-Naphthylamide
- PMID: 37240007
- PMCID: PMC10218020
- DOI: 10.3390/ijms24108662
Boosting the Antibacterial Activity of Azithromycin on Multidrug-Resistant Escherichia coli by Efflux Pump Inhibition Coupled with Outer Membrane Permeabilization Induced by Phenylalanine-Arginine β-Naphthylamide
Abstract
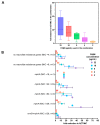
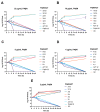
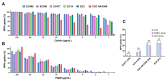
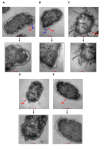
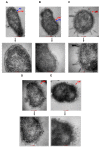
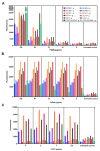
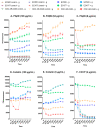
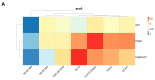
The global spread of multidrug-resistant (MDR) bacteria increases the demand for the discovery of new antibiotics and adjuvants. Phenylalanine-arginine β-naphthylamide (PAβN) is an inhibitor of efflux pumps in Gram-negative bacteria, such as the AcrAB-TolC complex in Escherichia coli. We aimed to explore the synergistic effect and mechanism of action of PAβN combined with azithromycin (AZT) on a group of MDR E. coli strains. Antibiotic susceptibility was tested for 56 strains, which were screened for macrolide resistance genes. Then, 29 strains were tested for synergy using the checkerboard assay. PAβN significantly enhanced AZT activity in a dose-dependent manner in strains expressing the mphA gene and encoding macrolide phosphotransferase, but not in strains carrying the ermB gene and encoding macrolide methylase. Early bacterial killing (6 h) was observed in a colistin-resistant strain with the mcr-1 gene, leading to lipid remodeling, which caused outer membrane (OM) permeability defects. Clear OM damage was revealed by transmission electron microscopy in bacteria exposed to high doses of PAβN. Increased OM permeability was also proven by fluorometric assays, confirming the action of PAβN on OM. PAβN maintained its activity as an efflux pump inhibitor at low doses without permeabilizing OM. A non-significant increase in acrA, acrB, and tolC expression in response to prolonged exposure to PAβN was noted in cells treated with PAβN alone or with AZT, as a reflection of bacterial attempts to counteract pump inhibition. Thus, PAβN was found to be effective in potentiating the antibacterial activity of AZT on E. coli through dose-dependent action. This warrants further investigations of its effect combined with other antibiotics on multiple Gram-negative bacterial species. Synergetic combinations will help in the battle against MDR pathogens, adding new tools to the arsenal of existing medications.
Keywords: Escherichia coli; azithromycin; efflux pump inhibitor; phenylalanine-arginine β-naphthylamide; synergy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Importance of Real-Time Assays To Distinguish Multidrug Efflux Pump-Inhibiting and Outer Membrane-Destabilizing Activities in Escherichia coli.J Bacteriol. 2015 Aug 1;197(15):2479-88. doi: 10.1128/JB.02456-14. Epub 2015 May 11. J Bacteriol. 2015. PMID: 25962916 Free PMC article.
-
Random mutagenesis of the multidrug transporter AcrB from Escherichia coli for identification of putative target residues of efflux pump inhibitors.Antimicrob Agents Chemother. 2014 Nov;58(11):6870-8. doi: 10.1128/AAC.03775-14. Epub 2014 Sep 2. Antimicrob Agents Chemother. 2014. PMID: 25182653 Free PMC article.
-
Discerning the role of polymyxin B nonapeptide in restoring the antibacterial activity of azithromycin against antibiotic-resistant Escherichia coli.Front Microbiol. 2022 Sep 21;13:998671. doi: 10.3389/fmicb.2022.998671. eCollection 2022. Front Microbiol. 2022. PMID: 36212888 Free PMC article.
-
Drug Efflux Pump Inhibitors: A Promising Approach to Counter Multidrug Resistance in Gram-Negative Pathogens by Targeting AcrB Protein from AcrAB-TolC Multidrug Efflux Pump from Escherichia coli.Biology (Basel). 2022 Sep 8;11(9):1328. doi: 10.3390/biology11091328. Biology (Basel). 2022. PMID: 36138807 Free PMC article. Review.
-
AcrB: a mean, keen, drug efflux machine.Ann N Y Acad Sci. 2020 Jan;1459(1):38-68. doi: 10.1111/nyas.14239. Epub 2019 Oct 6. Ann N Y Acad Sci. 2020. PMID: 31588569 Review.
Cited by
-
Targeting efflux pumps prevents the multi-step evolution of high-level resistance to fluoroquinolone in Pseudomonas aeruginosa.Microbiol Spectr. 2025 Apr;13(4):e0298124. doi: 10.1128/spectrum.02981-24. Epub 2025 Feb 21. Microbiol Spectr. 2025. PMID: 39982069 Free PMC article.
-
Harnessing Monoterpenes and Monoterpenoids as Weapons against Antimicrobial Resistance.Pol J Microbiol. 2025 Mar 7;74(1):1-18. doi: 10.33073/pjm-2025-010. eCollection 2025 Mar 1. Pol J Microbiol. 2025. PMID: 40052212 Free PMC article. Review.
-
Muropeptides and muropeptide transporters impact on host immune response.Gut Microbes. 2024 Jan-Dec;16(1):2418412. doi: 10.1080/19490976.2024.2418412. Epub 2024 Oct 22. Gut Microbes. 2024. PMID: 39439228 Free PMC article. Review.
-
Exploiting the fitness cost of metallo-β-lactamase expression can overcome antibiotic resistance in bacterial pathogens.Nat Microbiol. 2025 Jan;10(1):53-65. doi: 10.1038/s41564-024-01883-8. Epub 2025 Jan 2. Nat Microbiol. 2025. PMID: 39747690
-
Rational Design of Antimicrobial Peptides Based on Bacterial Type‑I Toxins AapA1, IbsC, and Fst1.ACS Omega. 2025 Jun 9;10(24):25790-25800. doi: 10.1021/acsomega.5c01863. eCollection 2025 Jun 24. ACS Omega. 2025. PMID: 40584331 Free PMC article.
References
-
- Lomovskaya O., Warren M.S., Lee A., Galazzo J., Fronko R., Lee M., Blais J., Cho D., Chamberland S., Renau T., et al. Identification and Characterization of Inhibitors of Multidrug Resistance Efflux Pumps in Pseudomonas aeruginosa: Novel Agents for Combination Therapy. Antimicrob. Agents Chemother. 2001;45:105–116. doi: 10.1128/AAC.45.1.105-116.2001. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

