Vitamin A Promotes the Fusion of Autophagolysosomes and Prevents Excessive Inflammasome Activation in Dextran Sulfate Sodium-Induced Colitis
- PMID: 37240022
- PMCID: PMC10218524
- DOI: 10.3390/ijms24108684
Vitamin A Promotes the Fusion of Autophagolysosomes and Prevents Excessive Inflammasome Activation in Dextran Sulfate Sodium-Induced Colitis
Abstract
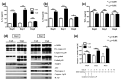
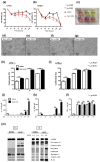
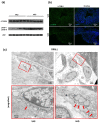
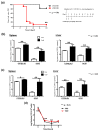
Vitamin A ensures intestinal homeostasis, impacting acquired immunity and epithelial barrier function; however, its role in innate immunity is mostly unknown. Here, we studied the impact of vitamin A in different dextran sulfate sodium (DSS)-induced colitis animal models. Interestingly, more severe DSS-induced colitis was observed in vitamin A-deficient (VAD) mice than in vitamin A-sufficient (VAS) mice; the same was observed in VAD severe combined immunodeficient mice lacking T/B cells. Remarkably, IL-1β production, LC3B-II expression, and inflammasome activity in the lamina propria were significantly elevated in VAD mice. Electron microscopy revealed numerous swollen mitochondria with severely disrupted cristae. In vitro, non-canonical inflammasome signaling-induced pyroptosis, LC3B-II and p62 expression, and mitochondrial superoxide levels were increased in murine macrophages (RAW 264.7) pretreated with retinoic acid receptor antagonist (Ro41-5253). These findings suggest that vitamin A plays a crucial role in the efficient fusion of autophagosomes with lysosomes in colitis.
Keywords: autophagy; inflammatory bowel disease; pyroptosis; retinoic acid; vitamin A.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Kastner T., Walsh K.K. The Optimal Evaluation and Treatment of Feeding Disorders in Children with Neurological Disorders. J. Am. Coll. Surg. 1995;181:383–386. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

