Hamster Sperm Possess Functional Na+/Ca2+-Exchanger 1: Its Implication in Hyperactivation
- PMID: 37240252
- PMCID: PMC10219178
- DOI: 10.3390/ijms24108905
Hamster Sperm Possess Functional Na+/Ca2+-Exchanger 1: Its Implication in Hyperactivation
Abstract
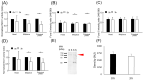
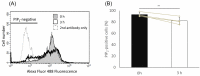
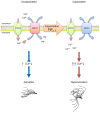
Previous studies demonstrated that hamster sperm hyperactivation is suppressed by extracellular Na+ by lowering intracellular Ca2+ levels, and Na+/Ca2+-exchanger (NCX) specific inhibitors canceled the suppressive effects of extracellular Na+. These results suggest the involvement of NCX in the regulation of hyperactivation. However, direct evidence of the presence and functionality of NCX in hamster spermatozoa is still lacking. This study aimed to reveal that NCX is present and is functional in hamster spermatozoa. First, NCX1 and NCX2 transcripts were detected via RNA-seq analyses of hamster testis mRNAs, but only the NCX1 protein was detected. Next, NCX activity was determined by measuring the Na+-dependent Ca2+ influx using the Ca2+ indicator Fura-2. The Na+-dependent Ca2+ influx was detected in hamster spermatozoa, notably in the tail region. The Na+-dependent Ca2+ influx was inhibited by the NCX inhibitor SEA0400 at NCX1-specific concentrations. NCX1 activity was reduced after 3 h of incubation in capacitating conditions. These results, together with authors' previous study, showed that hamster spermatozoa possesses functional NCX1 and that its activity was downregulated upon capacitation to trigger hyperactivation. This is the first study to successfully reveal the presence of NCX1 and its physiological function as a hyperactivation brake.
Keywords: NCX1; Na+/Ca2+ exchanger; sperm capacitation; sperm hyperactivation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Yanagimachi R. Mammalian fertilization. In: Knobil E., Neill. J.D., editors. Physiology of Reproduction. 2nd ed. Raven Press; New York, NY, USA: 1994. pp. 189–317.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

