PSEN1 E280A Cholinergic-like Neurons and Cerebral Spheroids Derived from Mesenchymal Stromal Cells and from Induced Pluripotent Stem Cells Are Neuropathologically Equivalent
- PMID: 37240306
- PMCID: PMC10218810
- DOI: 10.3390/ijms24108957
PSEN1 E280A Cholinergic-like Neurons and Cerebral Spheroids Derived from Mesenchymal Stromal Cells and from Induced Pluripotent Stem Cells Are Neuropathologically Equivalent
Abstract
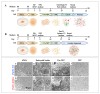
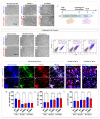
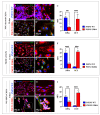
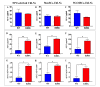
Alzheimer's disease (AD) is a chronic neurological condition characterized by the severe loss of cholinergic neurons. Currently, the incomplete understanding of the loss of neurons has prevented curative treatments for familial AD (FAD). Therefore, modeling FAD in vitro is essential for studying cholinergic vulnerability. Moreover, to expedite the discovery of disease-modifying therapies that delay the onset and slow the progression of AD, we depend on trustworthy disease models. Although highly informative, induced pluripotent stem cell (iPSCs)-derived cholinergic neurons (ChNs) are time-consuming, not cost-effective, and labor-intensive. Other sources for AD modeling are urgently needed. Wild-type and presenilin (PSEN)1 p.E280A fibroblast-derived iPSCs, menstrual blood-derived menstrual stromal cells (MenSCs), and umbilical cord-derived Wharton Jelly's mesenchymal stromal cells (WJ-MSCs) were cultured in Cholinergic-N-Run and Fast-N-Spheres V2 medium to obtain WT and PSEN 1 E280A cholinergic-like neurons (ChLNs, 2D) and cerebroid spheroids (CSs, 3D), respectively, and to evaluate whether ChLNs/CSs can reproduce FAD pathology. We found that irrespective of tissue source, ChLNs/CSs successfully recapitulated the AD phenotype. PSEN 1 E280A ChLNs/CSs show accumulation of iAPPβ fragments, produce eAβ42, present TAU phosphorylation, display OS markers (e.g., oxDJ-1, p-JUN), show loss of ΔΨm, exhibit cell death markers (e.g., TP53, PUMA, CASP3), and demonstrate dysfunctional Ca2+ influx response to ACh stimuli. However, PSEN 1 E280A 2D and 3D cells derived from MenSCs and WJ-MSCs can reproduce FAD neuropathology more efficiently and faster (11 days) than ChLNs derived from mutant iPSCs (35 days). Mechanistically, MenSCs and WJ-MSCs are equivalent cell types to iPSCs for reproducing FAD in vitro.
Keywords: Alzheimer; E280a; apoptosis; iPSCs; mesenchymal stromal; mutant; presenilin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Combination of Epigallocatechin-3-Gallate and Tramiprosate Prevent Accumulation of Intracellular Aβ and Dysfunctional Autophagy-Lysosomal Pathway at Earliest Stage of Transdifferentiation of Mesenchymal Stromal Cells into PSEN1 E280A Cholinergic-like Neurons.Int J Mol Sci. 2025 Apr 16;26(8):3756. doi: 10.3390/ijms26083756. Int J Mol Sci. 2025. PMID: 40332390 Free PMC article.
-
Cholinergic-like neurons carrying PSEN1 E280A mutation from familial Alzheimer's disease reveal intraneuronal sAPPβ fragments accumulation, hyperphosphorylation of TAU, oxidative stress, apoptosis and Ca2+ dysregulation: Therapeutic implications.PLoS One. 2020 May 21;15(5):e0221669. doi: 10.1371/journal.pone.0221669. eCollection 2020. PLoS One. 2020. PMID: 32437347 Free PMC article.
-
Cholinergic-like neurons and cerebral spheroids bearing the PSEN1 p.Ile416Thr variant mirror Alzheimer's disease neuropathology.Sci Rep. 2023 Aug 8;13(1):12833. doi: 10.1038/s41598-023-39630-4. Sci Rep. 2023. PMID: 37553376 Free PMC article.
-
Characteristics and clinical applications of Wharton's jelly-derived mesenchymal stromal cells.Curr Res Transl Med. 2020 Jan;68(1):5-16. doi: 10.1016/j.retram.2019.09.001. Epub 2019 Sep 19. Curr Res Transl Med. 2020. PMID: 31543433 Review.
-
Human Pluripotent Stem Cells as In Vitro Models of Neurodegenerative Diseases.Adv Exp Med Biol. 2020;1195:93-94. doi: 10.1007/978-3-030-32633-3_13. Adv Exp Med Biol. 2020. PMID: 32468463 Review.
Cited by
-
Combination of Epigallocatechin-3-Gallate and Tramiprosate Prevent Accumulation of Intracellular Aβ and Dysfunctional Autophagy-Lysosomal Pathway at Earliest Stage of Transdifferentiation of Mesenchymal Stromal Cells into PSEN1 E280A Cholinergic-like Neurons.Int J Mol Sci. 2025 Apr 16;26(8):3756. doi: 10.3390/ijms26083756. Int J Mol Sci. 2025. PMID: 40332390 Free PMC article.
-
Altering heparan sulfate suppresses cell abnormalities and neuron loss in Drosophila presenilin model of Alzheimer Disease.iScience. 2024 Jul 2;27(7):110256. doi: 10.1016/j.isci.2024.110256. eCollection 2024 Jul 19. iScience. 2024. PMID: 39109174 Free PMC article.
-
Induced Microglial-like Cells Derived from Familial and Sporadic Alzheimer's Disease Peripheral Blood Monocytes Show Abnormal Phagocytosis and Inflammatory Response to PSEN1 E280A Cholinergic-like Neurons.Int J Mol Sci. 2025 Jul 24;26(15):7162. doi: 10.3390/ijms26157162. Int J Mol Sci. 2025. PMID: 40806295 Free PMC article.
-
Combination of Tramiprosate, Curcumin, and SP600125 Reduces the Neuropathological Phenotype in Familial Alzheimer Disease PSEN1 I416T Cholinergic-like Neurons.Int J Mol Sci. 2024 Apr 30;25(9):4925. doi: 10.3390/ijms25094925. Int J Mol Sci. 2024. PMID: 38732141 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

