Mitochondrial Connexins and Mitochondrial Contact Sites with Gap Junction Structure
- PMID: 37240383
- PMCID: PMC10219162
- DOI: 10.3390/ijms24109036
Mitochondrial Connexins and Mitochondrial Contact Sites with Gap Junction Structure
Abstract
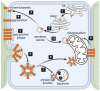
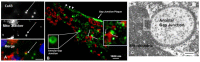
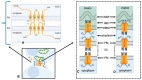
Mitochondria contain connexins, a family of proteins that is known to form gap junction channels. Connexins are synthesized in the endoplasmic reticulum and oligomerized in the Golgi to form hemichannels. Hemichannels from adjacent cells dock with one another to form gap junction channels that aggregate into plaques and allow cell-cell communication. Cell-cell communication was once thought to be the only function of connexins and their gap junction channels. In the mitochondria, however, connexins have been identified as monomers and assembled into hemichannels, thus questioning their role solely as cell-cell communication channels. Accordingly, mitochondrial connexins have been suggested to play critical roles in the regulation of mitochondrial functions, including potassium fluxes and respiration. However, while much is known about plasma membrane gap junction channel connexins, the presence and function of mitochondrial connexins remain poorly understood. In this review, the presence and role of mitochondrial connexins and mitochondrial/connexin-containing structure contact sites will be discussed. An understanding of the significance of mitochondrial connexins and their connexin contact sites is essential to our knowledge of connexins' functions in normal and pathological conditions, and this information may aid in the development of therapeutic interventions in diseases linked to mitochondria.
Keywords: annular gap junctions; connexin; contact sites; mitochondria.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
-
- Li H., Brodsky S., Kumari S., Valiunas V., Brink P., Kaide J., Nasjletti A., Goligorsky M.S. Paradoxical overexpression and translocation of connexin43 in homocysteine-treated endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2002;282:H2124–H2133. doi: 10.1152/ajpheart.01028.2001. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

