Development of Antibodies to Ustekinumab Is Associated with Loss of Response in Patients with Inflammatory Bowel Disease
- PMID: 37240501
- PMCID: PMC10219534
- DOI: 10.3390/jcm12103395
Development of Antibodies to Ustekinumab Is Associated with Loss of Response in Patients with Inflammatory Bowel Disease
Abstract
Monitoring of anti-drug antibodies in patients on ustekinumab is not routinely recommended in patients with inflammatory bowel disease (IBD) due to low rates of immunogenicity.
Aim of study: The purpose of this study was to investigate the relationship between anti-drug antibodies detected by a drug-tolerant assay and loss of response (LOR) to therapy in a cohort of patients with IBD being treated with ustekinumab.
Patients and methods: This retrospective study consecutively enrolled all adult patients with moderate to severe active IBD who had at least 2 years of follow-up after ustekinumab was initiated. LOR was defined as CDAI > 220 or HBI > 4 for Crohn's disease (CD) and partial Mayo subscore > 3 for ulcerative colitis (UC) and with a modification in disease management.
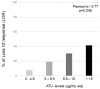
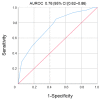
Results: Ninety patients were included (78 CD and 12 UC; mean age 37 years). Median levels of anti-ustekinumab antibodies (ATU) were significantly higher in patients with LOR compared to those with ongoing clinical response (15.2 µg/mL-eq CI (7.9-21.5) and 4.7 µg/mL-eq CI (2.1-10.5), respectively; p = 0.04). The area under the ROC curve (AUROC) for ATU in predicting LOR was 0.76. The optimal cut-off point for identifying patients with LOR was 9.5 µg/mL-eq with a sensitivity of 80% and specificity of 85%. Uni- and multivariate analyses showed that serum ATU ≥ 9.5 µg/mL-eq (hazard ratio (HR) 2.54, 95%CI (1.80-5.93)), p = 0.022, prior vedolizumab (HR 2.78, 95%CI (1.09-3.34), p = 0.019) and prior azathioprine (HR 0.54, 95%CI (0.20-0.76), p = 0.014) exposures were the only factors independently associated with LOR to UST.
Conclusion: In our real-life cohort, ATU was identified as an independent predictor of LOR to ustekinumab in patients with IBD.
Keywords: Crohn’s disease; drug-tolerant assay; immunogenicity; ustekinumab.
Conflict of interest statement
X. Roblin served as a speaker, a consultant and/or an advisory board member for MSD, Pfizer, Janssen, Takeda, Abbvie, Amgen, Biogen, Galapagos, Roche, Theradiag and celltrion. G. Duru: None. S. Kwiatek served as speaker for Celltrion. K. Papamichael received lecture/speaker fees from Mitsubishi Tanabe Pharma, Physicians Education Resource LLC and Grifols; scientific advisory board fees from ProciseDx Inc and Scipher Medicine Corporation; and serves as a consultant for Prometheus Laboratories Inc. A.S. Cheifetz served as a consultant and or advisory board member for Janssen, Abbvie, Protagonist, Spherix, Artizan (SAB), Food is Good, Clario, Pfizer, Fresenius Kabi, Artugen, Procise, Prometheus (SAB), Equillium, Samsung, Arena, Grifols, Bacainn, BMS, Takeda. S. Paul served as a speaker, a consultant and/or an advisory board member for MSD, Abbvie, Pfizer, Theradiag, Takeda. A.E. Berger: none. S. Nancey served as a speaker, a consultant and/or an advisory board member for MSD, Pfizer, Janssen, Takeda, Abbvie, Amgen, Biogen, Roche, Novartis. P. Veyrard: None. L. Waeckel: None.
Figures
References
-
- Kennedy N.A., Heap G.A., Green H.D., Hamilton B., Bewshea C., Walker G.J., Thomas A., Nice R., Perry M.H., Bouri S., et al. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn’s disease: A prospective, multicentre, cohort study. Lancet Gastroenterol. Hepatol. 2019;4:341–353. doi: 10.1016/S2468-1253(19)30012-3. - DOI - PubMed
-
- Casteele N.V., Herfarth H., Katz J., Falck-Ytter Y., Singh S. American Gastroenterological Association Institute Technical Review on the Role of Therapeutic Drug Monitoring in the Management of Inflammatory Bowel Diseases. Gastroenterology. 2017;153:835–857.e6. doi: 10.1053/j.gastro.2017.07.031. - DOI - PubMed
-
- Sandborn W.J., Feagan B.G., Fedorak R., Scherl E., Fleisher M.R., Katz S., Johanns J., Blank M., Rutgeerts P. A Randomized Trial of Ustekinumab, a Human Interleukin-12/23 Monoclonal Antibody, in Patients With Moderate-to-Severe Crohn’s Disease. Gastroenterology. 2008;135:1130–1141. doi: 10.1053/j.gastro.2008.07.014. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials