Sex Differences in Opioid Response Linked to OPRM1 and COMT genes DNA Methylation/Genotypes Changes in Patients with Chronic Pain
- PMID: 37240556
- PMCID: PMC10219447
- DOI: 10.3390/jcm12103449
Sex Differences in Opioid Response Linked to OPRM1 and COMT genes DNA Methylation/Genotypes Changes in Patients with Chronic Pain
Abstract
Analgesic-response variability in chronic noncancer pain (CNCP) has been reported due to several biological and environmental factors. This study was undertaken to explore sex differences linked to OPRM1 and COMT DNA methylation changes and genetic variants in analgesic response. A retrospective study with 250 real-world CNCP outpatients was performed in which data from demographic, clinical, and pharmacological variables were collected. DNA methylation levels (CpG island) were evaluated by pyrosequencing, and their interaction with the OPRM1 (A118G) and COMT (G472A) gene polymorphisms was studied. A priori-planned statistical analyses were conducted to compare responses between females and males. Sex-differential OPRM1 DNA methylation was observed to be linked to lower opioid use disorder (OUD) cases for females (p = 0.006). Patients with lower OPRM1 DNA methylation and the presence of the mutant G-allele reduced opioid dose requirements (p = 0.001), equal for both sexes. Moreover, COMT DNA methylation levels were negatively related to pain relief (p = 0.020), quality of life (p = 0.046), and some adverse events (probability > 90%) such as constipation, insomnia, or nervousness. Females were, significantly, 5 years older with high anxiety levels and a different side-effects distribution than males. The analyses demonstrated significant differences between females and males related to OPRM1 signalling efficiency and OUD, with a genetic-epigenetic interaction in opioid requirements. These findings support the importance of sex as a biological variable to be factored into chronic pain-management studies.
Keywords: DNA methylation; chronic pain; epigenetics; pharmacogenetics; sex differences.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
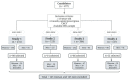
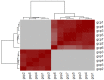



References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

