Evaluation of Long-Term Outcomes of Direct Acting Antiviral Agents in Chronic Kidney Disease Subjects: A Single Center Cohort Study
- PMID: 37240622
- PMCID: PMC10219487
- DOI: 10.3390/jcm12103513
Evaluation of Long-Term Outcomes of Direct Acting Antiviral Agents in Chronic Kidney Disease Subjects: A Single Center Cohort Study
Abstract
Background: The chronic kidney disease (CKD) population, including kidney transplant recipients (KTRs) and subjects on renal replacement therapy, is particularly vulnerable to unfavorable outcomes from chronic hepatitis C (CHC). Currently, there are oral direct-acting antiviral agents (DAAs) available to eradicate the virus with favorable short-term outcomes; however, their long-term effects are lacking. The aim of the study is to assess the long-term efficacy and safety of DAA therapy in the CKD population.
Methods: An observational, cohort single-center study was performed. Fifty-nine CHC subjects with CKD, treated with DAAs between 2016 and 2018, were enrolled in the study. Safety and efficacy profiles were assessed, including sustained virologic response (SVR), occult hepatitis C infection (OCI) incidence, and liver fibrosis.
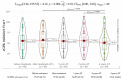
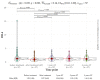
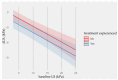
Results: SVR was achieved in 96% of cases (n = 57). OCI was diagnosed only in one subject following SVR. Significant liver stiffness regression was observed 4 years after SVR compared to baseline values (Mdn = 6.1 kPa, IQR = 3.75 kPa; 4.9 kPa, IQR = 2.9 kPa), p < 0.001. The most common adverse events were anemia, weakness, and urinary tract infection.
Conclusion: DAAs provide a safe and effective cure for CHC in both CKD patients and KTRs with a favorable safety profile in the long-term follow-up.
Keywords: chronic infection; direct-acting antiviral agents (DAA); hemodialysis; hepatitis C infection (HCV); kidney transplantation; liver fibrosis; occult hepatitis C infection; treatment efficacy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Detection of Occult Hepatitis C Virus Infection in Egyptian Patients Who Achieved a Sustained Virologic Response to Direct-Acting Antiviral Agents.Asian Pac J Cancer Prev. 2022 Sep 1;23(9):2965-2971. doi: 10.31557/APJCP.2022.23.9.2965. Asian Pac J Cancer Prev. 2022. PMID: 36172658 Free PMC article.
-
Gadolinium-ethoxybenzyl-diethylenetriamine penta-acetic acid-enhanced magnetic resonance imaging for evaluating fibrosis regression in chronic hepatitis C patients after direct-acting antiviral.World J Gastroenterol. 2022 May 28;28(20):2214-2226. doi: 10.3748/wjg.v28.i20.2214. World J Gastroenterol. 2022. PMID: 35721884 Free PMC article.
-
Non-alcoholic fatty liver disease is a risk factor for occurrence of hepatocellular carcinoma after sustained virologic response in chronic hepatitis C patients: A prospective four-years follow-up study.Metabol Open. 2021 Mar 26;10:100090. doi: 10.1016/j.metop.2021.100090. eCollection 2021 Jun. Metabol Open. 2021. PMID: 33889834 Free PMC article.
-
Hepatitis C virus and the kidney.Nat Rev Nephrol. 2019 Feb;15(2):73-86. doi: 10.1038/s41581-018-0081-8. Nat Rev Nephrol. 2019. PMID: 30455426 Review.
-
Sustained virologic response to direct-acting antiviral therapy in patients with chronic hepatitis C and hepatocellular carcinoma: A systematic review and meta-analysis.J Hepatol. 2019 Sep;71(3):473-485. doi: 10.1016/j.jhep.2019.04.017. Epub 2019 May 13. J Hepatol. 2019. PMID: 31096005
Cited by
-
Chronic Hepatitis C Infection Treated with Direct-Acting Antiviral Agents and Occurrence/Recurrence of Hepatocellular Carcinoma: Does It Still Matter?Viruses. 2024 Dec 10;16(12):1899. doi: 10.3390/v16121899. Viruses. 2024. PMID: 39772206 Free PMC article. Review.
-
Liver Stiffness Evaluation in Chronic Hepatitis C Patients with Cirrhosis before and after Direct-Acting Antivirals.Microorganisms. 2024 Jul 12;12(7):1418. doi: 10.3390/microorganisms12071418. Microorganisms. 2024. PMID: 39065186 Free PMC article. Review.
-
Renal Manifestations of Chronic Hepatitis C: A Review.J Clin Med. 2024 Sep 18;13(18):5536. doi: 10.3390/jcm13185536. J Clin Med. 2024. PMID: 39337023 Free PMC article. Review.
-
Real-world effectiveness of genotype-specific and pangenotypic direct-acting antivirals in HCV-infected patients with renal failure.Clin Exp Hepatol. 2023 Dec;9(4):320-334. doi: 10.5114/ceh.2023.133307. Epub 2023 Dec 15. Clin Exp Hepatol. 2023. PMID: 38774196 Free PMC article.
References
-
- Blach S., Zeuzem S., Manns M., Altraif I., Duberg A.-S., Muljono D.H., Waked I., Alavian S.M., Lee M.-H., Negro F., et al. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: A modelling study. Lancet Gastroenterol. Hepatol. 2017;2:161–176. doi: 10.1016/S2468-1253(16)30181-9. - DOI - PubMed
-
- Fissell R.B., Bragg-Gresham J.L., Woods J.D., Jadoul M., Gillespie B., Hedderwick S.A., Rayner H.C., Greenwood R.N., Akiba T., Young E.W. Patterns of hepatitis C prevalence and seroconversion in hemodialysis units from three continents: The DOPPS. Kidney Int. 2004;65:2335–2342. doi: 10.1111/j.1523-1755.2004.00649.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources

