A Real-World Prognosis in Idiopathic Pulmonary Fibrosis: A Special Reference to the Role of Antifibrotic Agents for the Elderly
- PMID: 37240670
- PMCID: PMC10219256
- DOI: 10.3390/jcm12103564
A Real-World Prognosis in Idiopathic Pulmonary Fibrosis: A Special Reference to the Role of Antifibrotic Agents for the Elderly
Abstract
Background: Idiopathic pulmonary fibrosis (IPF) is the most common and severe form of idiopathic interstitial pneumonia, and its prevalence increases with age. In the era of pre-antifibrotic agents, the median survival time of Japanese patients with IPF is 35 months, with a 5-year survival rate in western countries ranging from 20% to 40%. The prevalence of IPF is highest in elderly patients aged ≥75 years; however, the efficacy and safety of long-term use of pirfenidone and/or nintedanib are not fully understood.
Objective: This study aimed to determine the efficacy and safety of the sole use of antifibrotic agents (pirfenidone or nintendanib) for IPF in the elderly.
Method: We retrospectively reviewed patients with IPF who were diagnosed and treated with either pirfenidone or nintedanib in our hospital between 2008 and 2019. We excluded patients with the subsequent use of both antifibrotic agents. We examined the survival probability and frequency of acute exacerbation, with focus on long-term use (≥1 year), elderly patients (≥75 years of age), and disease severity.
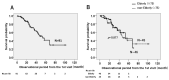
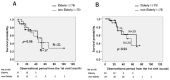
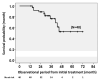
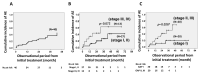
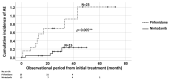
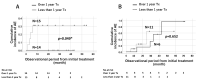
Results: We identified 91 patients with IPF (male to female ratio: 63 to 28, age 42 to 90 years). The numbers of patients with disease severity classified by JRS (I/II/III/IV) and GAP stage (I/II/III) were (38/6/17/20) and (39/36/6), respectively. The survival probabilities were comparable between the elderly (n = 46) and non-elderly groups (n = 45, p = 0.877). After the initiation of antifibrotic agents, the cumulative incidence ratio of acute exacerbation of IPF was significantly lower in the early stage (GAP stage I, n = 20) than in the progressive stage of disease (GAP stages II and III, n = 20, p = 0.028). A similar trend was noted in the JRS disease severity classification (I, II vs. III, IV) (n = 27 vs. n = 13, p = 0.072). In the long-term treatment (≥1 year) group (n = 40), the survival probabilities at 2 and 5 years after treatment initiation were 89.0% and 52.4%, respectively, which did not reach the median survival rate.
Conclusions: Even in elderly patients (≥75 years of age), antifibrotic agents demonstrated positive effects on survival probability and the frequency of acute exacerbation. These positive effects would be improved for earlier JRS/GAP stages or long-term use.
Keywords: IPF; acute exacerbation; antifibrotic agents; elderly patients; long-term treatment; survival probability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Natsuizaka M., Chiba H., Kuronuma K., Otsuka M., Kudo K., Mori M., Bando M., Sugiyama Y., Takahashi H. Epidemiologic survey of Japanese patients with idiopathic pulmonary fibrosis and investigation of ethnic differences. Am. J. Respir. Crit. Care Med. 2014;190:773–779. doi: 10.1164/rccm.201403-0566OC. - DOI - PubMed
-
- Sharif R. Overview of idiopathic pulmonary fibrosis (IPF) and evidence-based guidelines. Am. J. Manag. Care. 2017;23((Suppl. S11)):S176–S182. - PubMed
-
- Naccache J.M., Jouneau S., Didier M., Borie R., Cachanado M., Bourdin A., Reynaud-Gaubert M., Bonniaud P., Israël-Biet D., Prévot G., et al. Cyclophosphamide added to glucocorticoids in acute exacerbation of idiopathic pulmonary fibrosis (EXAFIP): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir. Med. 2022;10:26–34. doi: 10.1016/S2213-2600(21)00354-4. - DOI - PubMed
-
- Biondini D., Balestro E., Sverzellati N., Cocconcelli E., Bernardinello N., Ryerson C.J., Spagnolo P. Acute exacerbations of idiopathic pulmonary fibrosis (AE-IPF): An overview of current and future therapeutic strategies. Expert Rev. Respir. Med. 2020;14:405–414. doi: 10.1080/17476348.2020.1724096. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

