Efficacy and Safety of Neoadjuvant Luteinizing Hormone-Releasing Hormone Antagonist and Tegafur-Uracil Chemohormonal Therapy for High-Risk Prostate Cancer
- PMID: 37240717
- PMCID: PMC10223720
- DOI: 10.3390/life13051072
Efficacy and Safety of Neoadjuvant Luteinizing Hormone-Releasing Hormone Antagonist and Tegafur-Uracil Chemohormonal Therapy for High-Risk Prostate Cancer
Abstract
Background: This retrospective single-center cohort study evaluated the efficacy and safety of a combination of neoadjuvant luteinizing hormone-releasing hormone (LHRH) antagonist and tegafur-uracil (UFT) therapy (NCHT) and investigated the medical records of patients with high-risk PCa who underwent robot-assisted radical prostatectomy (RARP). The therapy was followed by RARP for high-risk PCa.
Materials and methods: The enrolled patients were divided into two groups: low-intermediate-risk PCa patients who underwent RARP without neoadjuvant therapy (non-high-risk) and those who underwent NCHT followed by RARP (high-risk group). This study enrolled 227 patients (126: non-high-risk and 101: high-risk group). Patients in the high-risk-group had high-grade cancer compared to those in the non-high-risk-group.
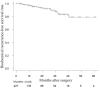
Results: At the median follow-up period of 12.0 months, there were no PCa deaths; two patients (0.9%) died of other causes. Twenty patients developed biochemical recurrence (BCR); the median time until BCR was 9.9 months after surgery. The 2-year biochemical recurrence-free survival rates were 94.2% and 91.1% in the non-high-risk and high-risk-group, respectively (p = 0.465). Grade ≥3 NCHT-related adverse events developed in nine patients (8.9%).
Conclusions: This study indicates that combining neoadjuvant LHRH antagonists and UFT followed by RARP may improve oncological outcomes in patients with high-risk PCa.
Keywords: high-risk prostate cancer; luteinizing hormone-releasing hormone antagonist; neoadjuvant chemohormonal therapy; robot-assisted radical prostatectomy; tegafur-uracil.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Combination approach using neoadjuvant therapy with radical prostatectomy for improving oncological outcomes of high-risk prostate cancer: a narrative review.Transl Cancer Res. 2024 Jul 31;13(7):3889-3897. doi: 10.21037/tcr-23-2394. Epub 2024 Jul 8. Transl Cancer Res. 2024. PMID: 39145084 Free PMC article. Review.
-
Neoadjuvant Chemohormonal Therapy Versus Pelvic Lymphadenectomy on Biochemical Recurrence in Patients with High- or Very-High-Risk Prostate Cancer Undergoing Robot-Assisted Radical Prostatectomy.Diseases. 2025 Mar 23;13(4):92. doi: 10.3390/diseases13040092. Diseases. 2025. PMID: 40277803 Free PMC article.
-
Efficacy and safety of neoadjuvant chemohormonal therapy for high-risk prostate cancer treated with robot-assisted laparoscopic radical prostatectomy: a propensity score-matched analysis (the MSUG94 group).Int Urol Nephrol. 2025 Mar;57(3):809-816. doi: 10.1007/s11255-024-04268-2. Epub 2024 Nov 8. Int Urol Nephrol. 2025. PMID: 39516348
-
Neoadjuvant chemohormonal therapy followed by robot-assisted and minimum incision endoscopic radical prostatectomy in patients with high-risk prostate cancer: comparison of perioperative and oncological outcomes at single institution.Int Urol Nephrol. 2018 Nov;50(11):1999-2005. doi: 10.1007/s11255-018-1985-8. Epub 2018 Sep 18. Int Urol Nephrol. 2018. PMID: 30229466
-
Neoadjuvant chemohormonal therapy before radical prostatectomy in high-risk prostate cancer: a mini-review.Am J Clin Exp Urol. 2024 Feb 15;12(1):1-7. eCollection 2024. Am J Clin Exp Urol. 2024. PMID: 38500864 Free PMC article. Review.
Cited by
-
Combination approach using neoadjuvant therapy with radical prostatectomy for improving oncological outcomes of high-risk prostate cancer: a narrative review.Transl Cancer Res. 2024 Jul 31;13(7):3889-3897. doi: 10.21037/tcr-23-2394. Epub 2024 Jul 8. Transl Cancer Res. 2024. PMID: 39145084 Free PMC article. Review.
-
Neoadjuvant Chemohormonal Therapy Versus Pelvic Lymphadenectomy on Biochemical Recurrence in Patients with High- or Very-High-Risk Prostate Cancer Undergoing Robot-Assisted Radical Prostatectomy.Diseases. 2025 Mar 23;13(4):92. doi: 10.3390/diseases13040092. Diseases. 2025. PMID: 40277803 Free PMC article.
-
Efficacy and safety of neoadjuvant chemohormonal therapy for high-risk prostate cancer treated with robot-assisted laparoscopic radical prostatectomy: a propensity score-matched analysis (the MSUG94 group).Int Urol Nephrol. 2025 Mar;57(3):809-816. doi: 10.1007/s11255-024-04268-2. Epub 2024 Nov 8. Int Urol Nephrol. 2025. PMID: 39516348
References
-
- Epstein J.I., Egevad L., Amin M.B., Delahunt B., Srigley J., Humphrey P.A., Grading Committee The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am. J. Surg. Pathol. 2016;40:244–252. doi: 10.1097/PAS.0000000000000530. - DOI - PubMed
-
- Prostate Cancer (2022) NCCN Guidelines®. [(accessed on 3 December 2022)]. Available online: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
-
- Mottet N., van den Bergh R.C.N., Briers E., Van den Broeck T., Cumberbatch M.G., De Santis M., Fanti S., Fossati N., Gandaglia G., Gillessen S., et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021;79:243–262. doi: 10.1016/j.eururo.2020.09.042. - DOI - PubMed
LinkOut - more resources
Full Text Sources

