Comparative Evaluation of Sildenafil Citrate and Estrogen as an Adjuvant Therapy for Treatment of Unexplained Infertility in Women
- PMID: 37241011
- PMCID: PMC10223516
- DOI: 10.3390/jpm13050842
Comparative Evaluation of Sildenafil Citrate and Estrogen as an Adjuvant Therapy for Treatment of Unexplained Infertility in Women
Abstract
Background: Uterine blood flow determines endometrial thickness. This study examined how vaginal sildenafil citrate and estradiol valerate altered endometrial thickness, blood flow, and fertility in infertile women.
Methods: This study observed 148 infertile women whose infertility was unexplained. Group 1 comprised 48 patients who received oral estradiol valerate (Cyclo-Progynova 2 mg/12 h white tablets) from day 6 till ovulation was initiated with clomiphene citrate. A number of 50 participants in group 2 received oral sildenafil (Respatio 20 mg/12 h film-coated tablets) for 5 days starting the day after their previous menstrual period and finishing on the day they ovulated with clomiphene citrate. Group 3 was the control group, with 50 patients receiving clomiphene citrate (Technovula 50 mg/12 h tablets) ovulation induction from the 2nd to 7th day of cycle. All patients had transvaginal ultrasounds to determine ovulation, follicle count, and fertility. Miscarriage, ectopic pregnancy, and multiple pregnancies were monitored for three months.
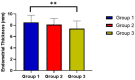
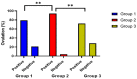
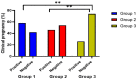
Results: The three groups' mean ETs differed statistically at p = 0.0004. A statistically significant difference was found between the three groups in terms of the number of follicles, with 69% of patients in group 1 having one and 31% having two or more, 76% of patients in group 2 having one and 24% having two or more, and 90% of patients in the control group having one and 10% having two or more (p = 0.038). The clinical pregnancy rates of the three groups were 58%, 46%, and 27%, respectively (p = 0.005). The distribution of all side effects was not statistically different between the three groups.
Conclusion: It is possible to claim that adding oral estrogen to clomiphene citrate therapy as an adjuvant therapy can improve endometrial thickness and, as a result, increase the pregnancy rates in unexplained infertility compared to sildenafil, especially in cases where the infertility has lasted less than two years. Most people who take sildenafil end up with a mild headache.
Keywords: clomiphene citrate; endometrial thickness; estradiol valerate; ovulation; pregnancy rates; sildenafil citrate; unexplained infertility.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- The European IVF-Monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE) Wyns C., De Geyter C., Calhaz-Jorge C., Kupka M.S., Motrenko T., Smeenk J., Bergh C., Tandler-Schneider A., Rugescu A.A., et al. ART in Europe, 2017: Results generated from European registries by ESHRE. Hum. Reprod. Open. 2021;2021:hoab026. - PubMed
-
- Zhang T., Li Z., Ren X., Huang B., Zhu G., Yang W., Jin L. Endometrial thickness as a predictor of the reproductive outcomes in fresh and frozen embryo transfer cycles: A retrospective cohort study of 1512 IVF cycles with morphologically good-quality blastocyst. Med. Baltim. 2018;97:e9689. doi: 10.1097/MD.0000000000009689. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

