Cleaning and Disinfecting Oval-Shaped Root Canals: Ex Vivo Evaluation of Three Rotary Instrumentation Systems with Passive Ultrasonic Irrigation
- PMID: 37241194
- PMCID: PMC10220979
- DOI: 10.3390/medicina59050962
Cleaning and Disinfecting Oval-Shaped Root Canals: Ex Vivo Evaluation of Three Rotary Instrumentation Systems with Passive Ultrasonic Irrigation
Abstract
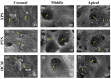
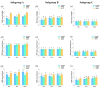
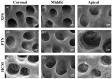
Background and Objectives: Successful root canal treatment depends on the thorough removal of biofilms through chemomechanical preparation. This study aimed to investigate and compare the cleaning and disinfecting efficiency of oval-shaped root canals using XP-endo Shaper (XPS), ProTaper Next (PTN), and HyFlex CM (HCM) in combination with passive ultrasonic irrigation (PUI). Materials and Methods: Ninety extracted teeth were contaminated and randomly divided into three groups: XPS, PTN, and HCM. Each group was assigned to three subgroups: subgroup A (sterile saline), subgroup B (3% sodium hypochlorite and 17% ethylenediaminetetraacetic acid), and subgroup C (3% sodium hypochlorite, 17% ethylenediaminetetraacetic acid, and PUI). Bacterial sampling was conducted both from baseline samples and samples after chemomechanical preparation. Scanning electron microscopy (SEM) was used to evaluate the residue bacterial biofilms, hard tissue debris, and smear layers on the buccolingual walls of oval-shaped root canals. Results: When combined with sterile saline, XPS demonstrated a higher reduction of bacterial counts and was more effective in eradicating Enterococcus faecalis in the middle third of the canals compared to the other instruments (p < 0.05). Additionally, when used with antimicrobial irrigants, XPS was more effective in disinfecting the coronal third of the canals than the other instruments (p < 0.05). Furthermore, XPS reduced hard tissue debris more effectively in the middle third of canals than in the apical third (p < 0.05). Conclusions: XPS outperforms PTN and HCM in disinfecting oval-shaped root canals. Despite the fact that combining XPS and PUI improves cleaning and disinfecting, removing hard tissue debris from the critical apical area remains challenging.
Keywords: endodontic disinfection; endodontic irrigation; oval-shaped root canal; passive ultrasonic irrigation; scanning electron microscope.
Conflict of interest statement
The authors deny any conflicts of interest in connection with this article.
Figures


Similar articles
-
Disinfecting oval-shaped root canals: effectiveness of different supplementary approaches.J Endod. 2011 Apr;37(4):496-501. doi: 10.1016/j.joen.2010.12.008. Epub 2011 Feb 25. J Endod. 2011. PMID: 21419297 Clinical Trial.
-
Comparative Evaluation of Smear Layer and Debris on the Canal Walls prepared with a Combination of Hand and Rotary ProTaper Technique using Scanning Electron Microscope.J Contemp Dent Pract. 2016 Jul 1;17(7):574-81. J Contemp Dent Pract. 2016. PMID: 27595725
-
The impact of different mechanical file systems with or without adjunctive approaches on disinfection of oval root canals: an ex vivo study.Br Dent J. 2025 Jul;239(1):53-60. doi: 10.1038/s41415-025-8504-y. Epub 2025 Jul 11. Br Dent J. 2025. PMID: 40646220 Free PMC article.
-
Effectiveness of XP-Endo Finisher and passive ultrasonic irrigation on intracanal medicament removal from root canals: a systematic review and meta-analysis.BMC Oral Health. 2021 Jun 9;21(1):294. doi: 10.1186/s12903-021-01644-7. BMC Oral Health. 2021. PMID: 34107959 Free PMC article.
-
Passive ultrasonic irrigation of the root canal: a review of the literature.Int Endod J. 2007 Jun;40(6):415-26. doi: 10.1111/j.1365-2591.2007.01243.x. Epub 2007 Apr 17. Int Endod J. 2007. PMID: 17442017 Review.
Cited by
-
Endodontic Retreatment of a Mandibular Second Molar With a C-shaped Root Canal Configuration: A Case Report.Cureus. 2024 Jan 23;16(1):e52812. doi: 10.7759/cureus.52812. eCollection 2024 Jan. Cureus. 2024. PMID: 38389597 Free PMC article.
-
Endodontic Treatment of Two Calcified Mandibular Central Incisors: A Case Report.Cureus. 2024 Jan 27;16(1):e53066. doi: 10.7759/cureus.53066. eCollection 2024 Jan. Cureus. 2024. PMID: 38410353 Free PMC article.
-
Primary and Re-exposure effects of D-enantiomeric peptide on metabolism, diversity, and composition of oral biofilms at different stages of recovery.Bioact Mater. 2025 Feb 20;48:257-272. doi: 10.1016/j.bioactmat.2025.02.023. eCollection 2025 Jun. Bioact Mater. 2025. PMID: 40060141 Free PMC article.
-
Evaluation of bacterial biofilm, smear layer, and debris removal efficacy of a hydro-dynamic cavitation system with physiological saline using a new ex vivo model: a CLSM and SEM study.BMC Oral Health. 2025 Jan 18;25(1):95. doi: 10.1186/s12903-025-05463-y. BMC Oral Health. 2025. PMID: 39827354 Free PMC article.
-
Endodontic Treatment of a Maxillary First Molar With Two Separate Palatal Roots: A Case Report.Cureus. 2024 Jan 8;16(1):e51907. doi: 10.7759/cureus.51907. eCollection 2024 Jan. Cureus. 2024. PMID: 38333498 Free PMC article.
References
-
- Siqueira J.F., Jr., Pérez A.R., Marceliano-Alves M.F., Provenzano J.C., Silva S.G., Pires F.R., Vieira G.C.S., Rocas I.N., Alves F.R.F. What happens to unprepared root canal walls: A correlative analysis using micro-computed tomography and histology/scanning electron microscopy. Int. Endod. J. 2018;51:501–508. doi: 10.1111/iej.12753. - DOI - PubMed
-
- Papic M., Papic M., Zivanovic S., Vuletic M., Zdravkovic D., Misic A., Miletic Kovacevic M., Popovic M. The prevalence of oval-shaped root canals: A morphometric study using cone-beam computed tomography and image analysis software. Aust. Endod. J. 2022;48:158–169. doi: 10.1111/aej.12554. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

