New Benzotrithiophene-Based Molecules as Organic P-Type Semiconductor for Small-Molecule Organic Solar Cells
- PMID: 37241386
- PMCID: PMC10222287
- DOI: 10.3390/ma16103759
New Benzotrithiophene-Based Molecules as Organic P-Type Semiconductor for Small-Molecule Organic Solar Cells
Abstract
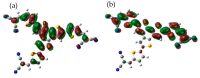
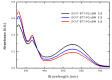
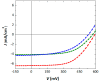
A new benzotrithiophene-based small molecule, namely 2,5,8-Tris[5-(2,2-dicyanovinyl)-2-thienyl]-benzo[1,2-b:3,4-b':6,5-b″]-trithiophene (DCVT-BTT), was successfully synthesized and subsequently characterized. This compound was found to present an intense absorption band at a wavelength position of ∼544 nm and displayed potentially relevant optoelectronic properties for photovoltaic devices. Theoretical studies demonstrated an interesting behavior of charge transport as electron donor (hole-transporting) active material for heterojunction cells. A preliminary study of small-molecule organic solar cells based on DCVT-BTT (as the P-type organic semiconductor) and phenyl-C61-butyric acid methyl ester (as the N-type organic semiconductor) exhibited a power conversion efficiency of 2.04% at a donor: acceptor weight ratio of 1:1.
Keywords: benzotrithiophene derivative; organic semiconductors; organic solar cells; small molecule; π-stacked assemblies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Rappaport P. The photovoltaic effect and its utilization. Sol. Energy. 1959;3:8–18. doi: 10.1016/0038-092X(59)90002-7. - DOI
-
- Saga T. Advances in crystalline silicon solar cell technology for industrial mass production. NPG Asia Mater. 2010;2:96–102. doi: 10.1038/asiamat.2010.82. - DOI
-
- De Wolf S., Descoeudres A., Holman Z., Ballif C. High-efficiency Silicon Heterojunction Solar Cells: A Review. Green. 2012;2:7–24. doi: 10.1515/green-2011-0018. - DOI
-
- Miles R., Zoppi G., Forbes I. Inorganic photovoltaic cells. Mater. Today. 2007;10:20. doi: 10.1016/S1369-7021(07)70275-4. - DOI
-
- Le Donne A., Scaccabarozzi A., Tombolato S., Marchionna S., Garattini P., Vodopivec B., Acciarri M., Binetti S. State of the Art and Perspectives of Inorganic Photovoltaics. ISRN Ren. Energy. 2013;2013:830731. doi: 10.1155/2013/830731. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

