In situ or Ex situ Synthesis for Electrochemical Detection of Hydrogen Peroxide-An Evaluation of Co2SnO4/RGO Nanohybrids
- PMID: 37241682
- PMCID: PMC10221292
- DOI: 10.3390/mi14051059
In situ or Ex situ Synthesis for Electrochemical Detection of Hydrogen Peroxide-An Evaluation of Co2SnO4/RGO Nanohybrids
Abstract
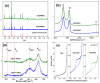
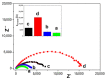
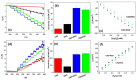
Nowadays, there is no doubt about the high electrocatalytic efficiency that is obtained when using hybrid materials between carbonaceous nanomaterials and transition metal oxides. However, the method to prepare them may involve differences in the observed analytical responses, making it necessary to evaluate them for each new material. The goal of this work was to obtain for the first time Co2SnO4 (CSO)/RGO nanohybrids via in situ and ex situ methods and to evaluate their performance in the amperometric detection of hydrogen peroxide. The electroanalytical response was evaluated in NaOH pH 12 solution using detection potentials of -0.400 V or 0.300 V for the reduction or oxidation of H2O2. The results show that for CSO there were no differences between the nanohybrids either by oxidation or by reduction, unlike what we previously observed with cobalt titanate hybrids, in which the in situ nanohybrid clearly had the best performance. On the other hand, no influence in the study of interferents and more stable signals were obtained when the reduction mode was used. In conclusion, for detecting hydrogen peroxide, any of the nanohybrids studied, i.e., in situ or ex situ, are suitable to be used, and more efficiency is obtained using the reduction mode.
Keywords: Co2SnO4; electrochemical detection; nanohybrids; reduced graphene oxide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Aguilar-Martínez J.A., Pech-Canul M.I., Esneider M., Toxqui A., Shaji S. Synthesis, Structure Parameter and Reaction Pathway for Spinel-Type Co2SnO4. Mater. Lett. 2012;78:28–31. doi: 10.1016/j.matlet.2012.03.042. - DOI
-
- Navrotsky A., Kleppa O.J. The Thermodynamics of Cation Distributions in Simple Spinels. J. Inorg. Nucl. Chem. 1967;29:2701–2714. doi: 10.1016/0022-1902(67)80008-3. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

