Anticancer Activity of Chalcones and Its Derivatives: Review and In Silico Studies
- PMID: 37241750
- PMCID: PMC10223217
- DOI: 10.3390/molecules28104009
Anticancer Activity of Chalcones and Its Derivatives: Review and In Silico Studies
Abstract
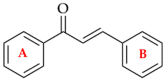
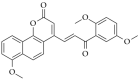
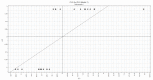
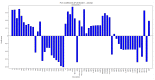
Chalcones are direct precursors in the biosynthesis of flavonoids. They have an α,β-unsaturated carbonyl system which gives them broad biological properties. Among the biological properties exerted by chalcones, their ability to suppress tumors stands out, in addition to their low toxicity. In this perspective, the present work explores the role of natural and synthetic chalcones and their anticancer activity in vitro reported in the last four years from 2019 to 2023. Moreover, we carried out a partial least square (PLS) analysis of the biologic data reported for colon adenocarcinoma lineage HCT-116. Information was obtained from the Web of Science database. Our in silico analysis identified that the presence of polar radicals such as hydroxyl and methoxyl contributed to the anticancer activity of chalcones derivatives. We hope that the data presented in this work will help researchers to develop effective drugs to inhibit colon adenocarcinoma in future works.
Keywords: anticancer activity; chalcones; drug discovery; in vitro; natural products; synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

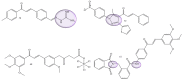
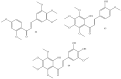
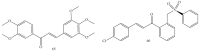
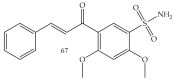


Similar articles
-
Natural Chalcones and Their Derivatives Target the Tumor Microenvironment in Colon Cancer.Crit Rev Immunol. 2022;42(6):27-39. doi: 10.1615/CritRevImmunol.2023047427. Crit Rev Immunol. 2022. PMID: 37082949 Review.
-
Anticancer Activity of Natural and Synthetic Chalcones.Int J Mol Sci. 2021 Oct 20;22(21):11306. doi: 10.3390/ijms222111306. Int J Mol Sci. 2021. PMID: 34768736 Free PMC article. Review.
-
Flavonoids, Chalcones, and Their Fluorinated Derivatives-Recent Advances in Synthesis and Potential Medical Applications.Molecules. 2025 May 30;30(11):2395. doi: 10.3390/molecules30112395. Molecules. 2025. PMID: 40509281 Free PMC article. Review.
-
Heterocyclic chalcone analogues as potential anticancer agents.Anticancer Agents Med Chem. 2013 Mar;13(3):422-32. Anticancer Agents Med Chem. 2013. PMID: 22721390 Review.
-
Design, Synthesis, and Evaluation of (2-(Pyridinyl)methylene)-1-tetralone Chalcones for Anticancer and Antimicrobial Activity.Med Chem. 2018;14(4):333-343. doi: 10.2174/1573406413666171020121244. Med Chem. 2018. PMID: 29065840
Cited by
-
AR71, Histamine H3 Receptor Ligand-In Vitro and In Vivo Evaluation (Anti-Inflammatory Activity, Metabolic Stability, Toxicity, and Analgesic Action).Int J Mol Sci. 2024 Jul 23;25(15):8035. doi: 10.3390/ijms25158035. Int J Mol Sci. 2024. PMID: 39125607 Free PMC article.
-
Bioprospecting, Synergistic Antifungal and Toxicological Aspects of the Hydroxychalcones and Their Association with Azole Derivates against Candida spp. for Treating Vulvovaginal Candidiasis.Pharmaceutics. 2024 Jun 21;16(7):843. doi: 10.3390/pharmaceutics16070843. Pharmaceutics. 2024. PMID: 39065540 Free PMC article.
-
Acridine-Based Chalcone 1C and ABC Transporters.Int J Mol Sci. 2025 Apr 27;26(9):4138. doi: 10.3390/ijms26094138. Int J Mol Sci. 2025. PMID: 40362377 Free PMC article.
-
(E)-2-Benzylidenecyclanones: Part XIX. Reaction of (E)-2-(4'-X-Benzylidene)-1-tetralones with Cellular Thiols: Comparison of Thiol Reactivities of Open-Chain Chalcones and Their Six- and Seven-Membered Cyclic Analogs.Int J Mol Sci. 2024 Jul 16;25(14):7773. doi: 10.3390/ijms25147773. Int J Mol Sci. 2024. PMID: 39063017 Free PMC article.
-
Synthesis of bis-Chalcones Based on Green Chemistry Strategies and Their Cytotoxicity Toward Human MeWo and A375 Melanoma Cell Lines.Molecules. 2024 Oct 31;29(21):5171. doi: 10.3390/molecules29215171. Molecules. 2024. PMID: 39519811 Free PMC article.
References
-
- Neto R.A.d.M., Santos C.B.R., Henriques S.V.C., Machado L.d.O., Cruz J.N., da Silva C.H.T.d.P., Federico L.B., de Oliveira E.H.C., de Souza M.P.C., da Silva P.N.B. Novel Chalcones Derivatives with Potential Antineoplastic Activity Investigated by Docking and Molecular Dynamics Simulations. J. Biomol. Struct. Dyn. 2022;40:2204–2216. doi: 10.1080/07391102.2020.1839562. - DOI - PubMed
-
- Helmy M.T., Sroor F.M., Mahrous K.F., Mahmoud K., Hassaneen H.M., Saleh F.M., Abdelhamid I.A., Mohamed Teleb M.A. Anticancer Activity of Novel 3-(Furan-2-yl) Pyrazolyl and 3-(Thiophen-2-yl) Pyrazolyl Hybrid Chalcones: Synthesis and In Vitro Studies. Arch. Pharm. 2022;355:2100381. doi: 10.1002/ardp.202100381. - DOI - PubMed
-
- Rioux B., Pouget C., Ndong-Ntoutoume G.M.A., Granet R., Gamond A., Laurent A., Pinon A., Champavier Y., Liagre B., Fagnère C. Enhancement of Hydrosolubility and In Vitro Antiproliferative Properties of Chalcones Following Encapsulation into β-Cyclodextrin/Cellulose-Nanocrystal Complexes. Bioorganic Med. Chem. Lett. 2019;29:1895–1898. doi: 10.1016/j.bmcl.2019.05.056. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- Financing Code 001/Coordenação de Aperfeicoamento de Pessoal de Nível Superior
- #431254/2018-4, #309648/2019-0, #308590/2017-1, #306798/2020-4/National Council for Scientific and Technological Development
- funding No. 04/2022 - grant number #3783/2022-0/Fundação de Apoio à Pesquisa do Estado da Paraíba
LinkOut - more resources
Full Text Sources

