PROTACs: Emerging Targeted Protein Degradation Approaches for Advanced Druggable Strategies
- PMID: 37241755
- PMCID: PMC10224132
- DOI: 10.3390/molecules28104014
PROTACs: Emerging Targeted Protein Degradation Approaches for Advanced Druggable Strategies
Abstract
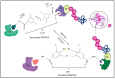
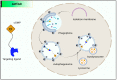
A potential therapeutic strategy to treat conditions brought on by the aberrant production of a disease-causing protein is emerging for targeted protein breakdown using the PROTACs technology. Few medications now in use are tiny, component-based and utilize occupancy-driven pharmacology (MOA), which inhibits protein function for a short period of time to temporarily alter it. By utilizing an event-driven MOA, the proteolysis-targeting chimeras (PROTACs) technology introduces a revolutionary tactic. Small-molecule-based heterobifunctional PROTACs hijack the ubiquitin-proteasome system to trigger the degradation of the target protein. The main challenge PROTAC's development facing now is to find potent, tissue- and cell-specific PROTAC compounds with favorable drug-likeness and standard safety measures. The ways to increase the efficacy and selectivity of PROTACs are the main focus of this review. In this review, we have highlighted the most important discoveries related to the degradation of proteins by PROTACs, new targeted approaches to boost proteolysis' effectiveness and development, and promising future directions in medicine.
Keywords: PROTACs; degradation; druggable technologies; emerging medicine; proteolysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
Publication types
MeSH terms
Substances
Grants and funding
- 21 YF5FA126/Key R&D project of Gansu Provincial Department of science and technology "Development of proteomic Detection and Diagnosis Prediction Model for Gout Based on Mass spectrometry"
- YJS-BD-23/Establishment of molecular diagnostic methods for stroke susceptibility genes and study of molecular epidemic disease
LinkOut - more resources
Full Text Sources

