Synthesis and Antiproliferative Insights of Lipophilic Ru(II)-Hydroxy Stearic Acid Hybrid Species
- PMID: 37241793
- PMCID: PMC10222286
- DOI: 10.3390/molecules28104051
Synthesis and Antiproliferative Insights of Lipophilic Ru(II)-Hydroxy Stearic Acid Hybrid Species
Abstract
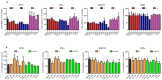
Metallodrugs represent a combination of multifunctionalities that are present concomitantly and can act differently on diverse biotargets. Their efficacy is often related to the lipophilic features exhibited both by long carbo-chains and the phosphine ligands. Three Ru(II) complexes containing hydroxy stearic acids (HSAs) were successfully synthesized in order to evaluate possible synergistic effects between the known antitumor activity of HSA bio-ligands and the metal center. HSAs were reacted with [Ru(H)2CO(PPh3)3] selectively affording O,O-carboxy bidentate complexes. The organometallic species were fully characterized spectroscopically using ESI-MS, IR, UV-Vis, and NMR techniques. The structure of the compound Ru-12-HSA was also determined using single crystal X-ray diffraction. The biological potency of ruthenium complexes (Ru-7-HSA, Ru-9-HSA, and Ru-12-HSA) was studied on human primary cell lines (HT29, HeLa, and IGROV1). To obtain detailed information about anticancer properties, tests for cytotoxicity, cell proliferation, and DNA damage were performed. The results demonstrate that the new ruthenium complexes, Ru-7-HSA and Ru-9-HSA, possess biological activity. Furthermore, we observed that the Ru-9-HSA complex shows increased antitumor activity on colon cancer cells, HT29.
Keywords: DNA damage; Ru(II); alkyl chain; anticancer; hydroxy stearic acid; lipophilicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
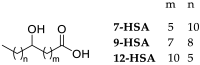




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

