Novel Approach to the Construction of Fused Indolizine Scaffolds: Synthesis of Rosettacin and the Aromathecin Family of Compounds
- PMID: 37241799
- PMCID: PMC10222911
- DOI: 10.3390/molecules28104059
Novel Approach to the Construction of Fused Indolizine Scaffolds: Synthesis of Rosettacin and the Aromathecin Family of Compounds
Abstract
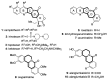
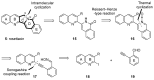
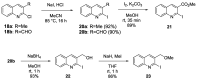
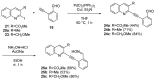
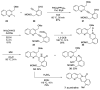
Camptothecin-like compounds are actively employed as anticancer drugs in clinical treatments. The aromathecin family of compounds, which contains the same indazolidine core structure as the camptothecin family of compounds, is also expected to display promising anticancer activity. Therefore, the development of a suitable and scalable synthetic method of aromathecin synthesis is of great research interest. In this study, we report the development of a new synthetic approach for constructing the pentacyclic scaffold of the aromathecin family by forming the indolizidine moiety after synthesizing the isoquinolone moiety. Thermal cyclization of 2-alkynylbenzaldehyde oxime to the isoquinoline N-oxide, followed by a Reissert-Henze-type reaction, forms the key strategy in this isoquinolone synthesis. Under the optimum reaction conditions for the Reissert-Henze-type reaction step, microwave irradiation-assisted heating of the purified N-oxide in acetic anhydride at 50 °C reduced the formation of the 4-acetoxyisoquinoline byproduct to deliver the desired isoquinolone at a 73% yield after just 3.5 h. The eight-step sequence employed afforded rosettacin (simplest member of the aromathecin family) at a 23.8% overall yield. The synthesis of rosettacin analogs was achieved by applying the developed strategy and may be generally applicable to the production of other fused indolizidine compounds.
Keywords: 22-hydroxyacuminatine; acuminatine; benz[6,7]indolizino[1,2-b]quinolin-11(13H)-one; thermal cyclization; total synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



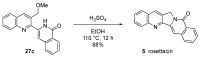
Similar articles
-
Total Synthesis and Biological Evaluation of 22-Hydroxyacuminatine and the Related Natural Products Norketoyobyrine and Naucleficine.Molecules. 2025 Jun 19;30(12):2650. doi: 10.3390/molecules30122650. Molecules. 2025. PMID: 40572614 Free PMC article.
-
Recent Advances in the Synthesis of Rosettacin.Molecules. 2024 May 7;29(10):2176. doi: 10.3390/molecules29102176. Molecules. 2024. PMID: 38792039 Free PMC article. Review.
-
Progress on the Synthesis of the Aromathecin Family of Compounds: An Overview.Molecules. 2024 May 18;29(10):2380. doi: 10.3390/molecules29102380. Molecules. 2024. PMID: 38792241 Free PMC article. Review.
-
Synthesis of the parent and substituted tetracyclic ABCD ring cores of camptothecins via 1-(3-aryl-2-propynyl)- 1,6-dihydro-6-oxo-2-pyridinecarbonitriles.Org Lett. 2006 Sep 28;8(20):4665-7. doi: 10.1021/ol0620242. Org Lett. 2006. PMID: 16986976
-
Enantioselective synthesis of (--)-gilbertine via a cationic cascade cyclization.J Am Chem Soc. 2004 Mar 24;126(11):3534-8. doi: 10.1021/ja0399021. J Am Chem Soc. 2004. PMID: 15025481
Cited by
-
Total Synthesis and Biological Evaluation of 22-Hydroxyacuminatine and the Related Natural Products Norketoyobyrine and Naucleficine.Molecules. 2025 Jun 19;30(12):2650. doi: 10.3390/molecules30122650. Molecules. 2025. PMID: 40572614 Free PMC article.
-
Indole Derivatives: Unveiling New Frontiers in Medicinal and Synthetic Organic Chemistry.Molecules. 2023 Jul 18;28(14):5477. doi: 10.3390/molecules28145477. Molecules. 2023. PMID: 37513349 Free PMC article.
-
Efficient Solvent-Free Synthesis of Indolizines Using CuBr Catalyst from Pyridine, Acetophenone, and Electron-Deficient Alkenes.Molecules. 2024 Apr 29;29(9):2061. doi: 10.3390/molecules29092061. Molecules. 2024. PMID: 38731552 Free PMC article.
-
Recent Advances in the Synthesis of Rosettacin.Molecules. 2024 May 7;29(10):2176. doi: 10.3390/molecules29102176. Molecules. 2024. PMID: 38792039 Free PMC article. Review.
-
Progress on the Synthesis of the Aromathecin Family of Compounds: An Overview.Molecules. 2024 May 18;29(10):2380. doi: 10.3390/molecules29102380. Molecules. 2024. PMID: 38792241 Free PMC article. Review.
References
-
- Wall M.E., Wani M.C., Cook C.E., Palmer K.H., McPhail A.T., Sim G.A. Plant Antitumor Agents. I. The Isolation and Structure of Camptothecin, a Novel Alkaloidal Leukemia and Tumor Inhibitor from Camptotheca acuminata. J. Am. Chem. Soc. 1966;88:3888–8890. doi: 10.1021/ja00968a057. - DOI
-
- Walraven H.G.M., Pandit U.K. A facile two synthon approach to the camptothecin skeleton. Tetrahedron. 1980;36:321–327. doi: 10.1016/0040-4020(80)80022-6. - DOI
-
- Pinho P.M.M., Pinto M.M.M., Kijjoa A., Pharadai K., Díaz J.G., Herz W. Protoberberine alkaloids from Coscinium fenestratum. Phytochemistry. 1992;31:1403–1407. doi: 10.1016/0031-9422(92)80301-T. - DOI
-
- Pailee P., Prachyawarakorn V., Mahidol C., Ruchirawat S., Kittakoop P. Protoberberine Alkaloids and Cancer Chemopreventive Properties of Compounds from Alangium salviifolium. Eur. J. Org. Chem. 2011;2011:3809–3814. doi: 10.1002/ejoc.201100423. - DOI
-
- Kawato Y., Aonuma M., Hirota Y., Kuga H., Sato K. Intracellular Roles of SN-38, a Metabolite of the Camptothecin Derivative CPT-11, in the Antitumor Effect of CPT-11. Cancer Res. 1991;51:4187–4197. - PubMed
LinkOut - more resources
Full Text Sources

