Biocytin-Labeling in Whole-Cell Recording: Electrophysiological and Morphological Properties of Pyramidal Neurons in CYLD-Deficient Mice
- PMID: 37241833
- PMCID: PMC10221440
- DOI: 10.3390/molecules28104092
Biocytin-Labeling in Whole-Cell Recording: Electrophysiological and Morphological Properties of Pyramidal Neurons in CYLD-Deficient Mice
Abstract
Biocytin, a chemical compound that is an amide formed from the vitamin biotin and the amino acid L-lysine, has been used as a histological dye to stain nerve cells. Electrophysiological activity and morphology are two key characteristics of neurons, but revealing both the electrophysiological and morphological properties of the same neuron is challenging. This article introduces a detailed and easy-to-operate procedure for single-cell labeling in combination with whole-cell patch-clamp recording. Using a recording electrode filled with a biocytin-containing internal solution, we demonstrate the electrophysiological and morphological characteristics of pyramidal (PNs), medial spiny (MSNs) and parvalbumin neurons (PVs) in brain slices, where the electrophysiological and morphological properties of the same individual cell are elucidated. We first introduce a protocol for whole-cell patch-clamp recording in various neurons, coupled with the intracellular diffusion of biocytin delivered by the glass capillary of the recording electrode, followed by a post hoc procedure to reveal the architecture and morphology of biocytin-labeled neurons. An analysis of action potentials (APs) and neuronal morphology, including the dendritic length, number of intersections, and spine density of biocytin-labeled neurons, were performed using ClampFit and Fiji Image (ImageJ), respectively. Next, to take advantage of the techniques introduced above, we uncovered defects in the APs and the dendritic spines of PNs in the primary motor cortex (M1) of deubiquitinase cylindromatosis (CYLD) knock-out (Cyld-/-) mice. In summary, this article provides a detailed methodology for revealing the morphology as well as the electrophysiological activity of a single neuron that will have many applications in neurobiology.
Keywords: CYLD; action potential; biocytin; dendritic spine; single-cell labeling; whole-cell patch-clamp recording.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

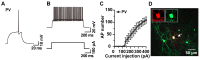
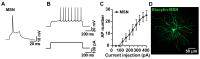
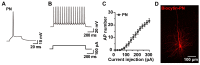
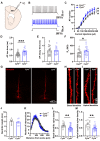
References
-
- Gouwens N.W., Sorensen S.A., Berg J., Lee C., Jarsky T., Ting J., Sunkin S.M., Feng D., Anastassiou C.A., Barkan E., et al. Classification of electrophysiological and morphological neuron types in the mouse visual cortex. Nat. Neurosci. 2019;22:1182–1195. doi: 10.1038/s41593-019-0417-0. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

