Rovibrational Spectroscopy of Trans and Cis Conformers of 2-Furfural from High-Resolution Fourier Transform and QCL Infrared Measurements
- PMID: 37241905
- PMCID: PMC10224235
- DOI: 10.3390/molecules28104165
Rovibrational Spectroscopy of Trans and Cis Conformers of 2-Furfural from High-Resolution Fourier Transform and QCL Infrared Measurements
Abstract
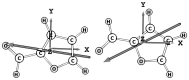
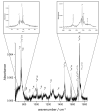
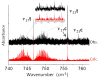
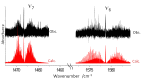
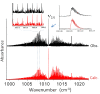
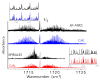
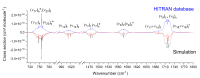
The ortho-isomer 2-furfural (2-FF), which is a primary atmospheric pollutant produced from biomass combustion, is also involved in oxidation processes leading to the formation of secondary organic aerosols. Its contribution to radiative forcing remains poorly understood. Thus, monitoring 2-FF directly in the atmosphere or in atmospheric simulation chambers to characterize its reactivity is merited. The present study reports an extensive jet-cooled rovibrational study of trans and cis conformers of 2-FF in the mid-IR region using two complementary setups: a continuous supersonic jet coupled to a high-resolution Fourier transform spectrometer on the IR beamline of the SOLEIL synchrotron (JET-AILES), and a pulsed jet coupled to a mid-IR tunable quantum cascade laser spectrometer (SPIRALES). Firstly, jet-cooled spectra recorded at rotational temperatures ranging between 20 and 50 K were exploited to derive reliable excited-state molecular parameters of trans- and cis-2-FF vibrational bands in the fingerprint region. The parameters were obtained from global fits of 11,376 and 3355 lines distributed over eight and three vibrational states (including the ground state), respectively, with a root mean square of 12 MHz. In a second step, the middle resolution spectrum of 2-FF recorded at 298.15 K and available in the HITRAN database was reconstructed by extrapolating the data derived from our low-temperature high-resolution analyses to determine the cross sections of each vibrational band of both 2-FF conformers in the 700-1800 cm-1 region. Finally, we clearly demonstrated that the contribution of hot bands observed in the room temperature 2-FF spectrum, estimated between 40 and 63% of the fundamental band, must be imperatively introduced in our simulation to correctly reproduce the HITRAN vibrational cross sections of 2-FF with a deviation smaller than 10%.
Keywords: QCL source; furfural; jet-cooling; rovibrational spectroscopy; synchrotron-based FTIR spectroscopy; vibrational cross section.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Edye L.A., Richards G.N. Analysis of Condensates from Wood Smoke: Components Derived from Polysaccharides and Lignins. Environ. Sci. Technol. 1991;25:1133–1137. doi: 10.1021/es00018a018. - DOI
-
- Andreae M.O., Merlet P. Emission of trace gases and aerosols from biomass burning. Glob. Biogeochem. Cycles. 2001;15:955–966. doi: 10.1029/2000GB001382. - DOI
-
- Paczkowski S., Paczkowska M., Dippel S., Schulze N., Schütz S., Sauerwald T., Weiß A., Bauer M., Gottschald J., Kohl C.D. The olfaction of a fire beetle leads to new concepts for early fire warning systems. Sens. Actuators B Chem. 2013;183:273–282. doi: 10.1016/j.snb.2013.03.123. - DOI
-
- Colmenar I., Martín P., Cabañas B., Salgado S., Villanueva F., Ballesteros B. Evaluation of the SOA Formation in the Reaction of Furfural with Atmospheric Oxidants. Atmosphere. 2020;11:927. doi: 10.3390/atmos11090927. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

