Essential Amino Acids-Rich Diet Increases Cardiomyocytes Protection in Doxorubicin-Treated Mice
- PMID: 37242170
- PMCID: PMC10222879
- DOI: 10.3390/nu15102287
Essential Amino Acids-Rich Diet Increases Cardiomyocytes Protection in Doxorubicin-Treated Mice
Abstract
Background: Doxorubicin (Doxo) is a widely prescribed drug against many malignant cancers. Unfortunately, its utility is limited by its toxicity, in particular a progressive induction of congestive heart failure. Doxo acts primarily as a mitochondrial toxin, with consequent increased production of reactive oxygen species (ROS) and attendant oxidative stress, which drives cardiac dysfunction and cell death. A diet containing a special mixture of all essential amino acids (EAAs) has been shown to increase mitochondriogenesis, and reduce oxidative stress both in skeletal muscle and heart. So, we hypothesized that such a diet could play a favorable role in preventing Doxo-induced cardiomyocyte damage.
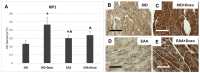
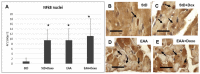
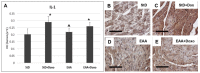
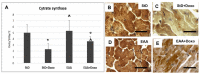
Methods: Using transmission electron microscopy, we evaluated cells' morphology and mitochondria parameters in adult mice. In addition, by immunohistochemistry, we evaluated the expression of pro-survival marker Klotho, as well as markers of necroptosis (RIP1/3), inflammation (TNFα, IL1, NFkB), and defense against oxidative stress (SOD1, glutathione peroxidase, citrate synthase).
Results: Diets with excess essential amino acids (EAAs) increased the expression of Klotho and enhanced anti-oxidative and anti-inflammatory responses, thereby promoting cell survival.
Conclusion: Our results further extend the current knowledge about the cardioprotective role of EAAs and provide a novel theoretical basis for their preemptive administration to cancer patients undergoing chemotherapy to alleviate the development and severity of Doxo-induced cardiomyopathy.
Keywords: Klotho; doxorubicin; essential amino acids; heart; mice; necroptosis.
Conflict of interest statement
The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures







References
-
- Hortobagyi G.N. Anthracyclines in the treatment of cancer: An overview. Drugs. 1997;54:1–7. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

