Comparative Analysis of Fecal Microbiota in Vegetarians and Omnivores
- PMID: 37242241
- PMCID: PMC10221195
- DOI: 10.3390/nu15102358
Comparative Analysis of Fecal Microbiota in Vegetarians and Omnivores
Abstract
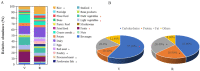
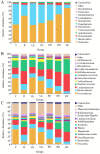
Diet has a significant impact on fecal microbiota, which in turn plays an important role in human health. To evaluate the impact of dietary habits on fecal microbiota, we investigated the fecal microbial composition in vegetarians and omnivores using 16S rRNA gene sequencing, and estimated the correlation between fecal microbiota, body mass and diet. The dietary data showed that vegetarians consumed more plant-based foods rich in dietary fiber, omnivores consumed more animal-based foods rich in fat and overweight and obese people consumed more high-energy foods. Compared to omnivores, vegetarians had greater richness and diversity in their fecal microbiota. The Firmicutes/Bacteroidetes ratio was lower and the Prevotella/Bacteroides ratio was higher in vegetarians. The meat intake correlated positively with the proportion of Bacteroides and negatively with the proportion of Prevotella. The composition and diversity in fecal microbiota in the normal weight group, overweight group and obesity group were similar to that of vegetarians and omnivores, respectively. This paper revealed the distinctive characteristics of fecal microbiota in vegetarians and omnivores. The omnivorous diet contained more fat, which reduced the fecal microbial diversity, and was more likely to lead to being overweight or obese.
Keywords: fecal microbiota; obesity; omnivores; vegetarians.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Federici S., Kredo-Russo S., Valdés-Mas R., Kviatcovsky D., Weinstock E., Matiuhin Y., Silberberg Y., Atarashi K., Furuichi M., Oka X., et al. Targeted suppression of human IBD-associated gut microbiota commensals by phage consortia for treatment of intestinal inflammation. Cell. 2022;185:2879–2898. doi: 10.1016/j.cell.2022.07.003. - DOI - PubMed
-
- Wang Z., Usyk M., Vázquez-Baeza Y., Chen G.C., Isasi C.R., Williams-Nguyen J.S., Hua S., McDonald D., Thyagarajan B., Daviglus M.L., et al. Microbial co-occurrence complicates associations of gut microbiome with US immigration, dietary intake and obesity. Genome Biol. 2021;22:336. doi: 10.1186/s13059-021-02559-w. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

