The Inherited Intestinal Microbiota from Myeloid-Specific ZIP8KO Mice Impairs Pulmonary Host Defense against Pneumococcal Pneumonia
- PMID: 37242309
- PMCID: PMC10222741
- DOI: 10.3390/pathogens12050639
The Inherited Intestinal Microbiota from Myeloid-Specific ZIP8KO Mice Impairs Pulmonary Host Defense against Pneumococcal Pneumonia
Abstract
Intestinal dysbiosis increases susceptibility to infection through the alteration of metabolic profiles, which increases morbidity. Zinc (Zn) homeostasis in mammals is tightly regulated by 24 Zn transporters. ZIP8 is unique in that it is required by myeloid cells to maintain proper host defense against bacterial pneumonia. In addition, a frequently occurring ZIP8 defective variant (SLC39A8 rs13107325) is strongly associated with inflammation-based disorders and bacterial infection. In this study, we developed a novel model to study the effects of ZIP8-mediated intestinal dysbiosis on pulmonary host defense independent of the genetic effects. Cecal microbial communities from a myeloid-specific Zip8 knockout mouse model were transplanted into germ-free mice. Conventionalized ZIP8KO-microbiota mice were then bred to produce F1 and F2 generations of ZIP8KO-microbiota mice. F1 ZIP8KO-microbiota mice were also infected with S. pneumoniae, and pulmonary host defense was assessed. Strikingly, the instillation of pneumococcus into the lung of F1 ZIP8KO-microbiota mice resulted in a significant increase in weight loss, inflammation, and mortality when compared to F1 wild-type (WT)-microbiota recipients. Similar defects in pulmonary host defense were observed in both genders, although consistently greater in females. From these results, we conclude that myeloid Zn homeostasis is not only critical for myeloid function but also plays a significant role in the maintenance and control of gut microbiota composition. Further, these data demonstrate that the intestinal microbiota, independent of host genetics, play a critical role in governing host defense in the lung against infection. Finally, these data strongly support future microbiome-based interventional studies, given the high incidence of zinc deficiency and the rs13107325 allele in humans.
Keywords: gut-lung axis; host defense; microbiome; pneumonia; zinc transporter.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






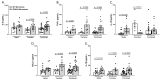

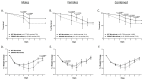

Similar articles
-
ZIP8-Mediated Intestinal Dysbiosis Impairs Pulmonary Host Defense against Bacterial Pneumonia.Int J Mol Sci. 2022 Jan 18;23(3):1022. doi: 10.3390/ijms23031022. Int J Mol Sci. 2022. PMID: 35162945 Free PMC article.
-
Critical Role of Zinc Transporter (ZIP8) in Myeloid Innate Immune Cell Function and the Host Response against Bacterial Pneumonia.J Immunol. 2021 Sep 1;207(5):1357-1370. doi: 10.4049/jimmunol.2001395. Epub 2021 Aug 11. J Immunol. 2021. PMID: 34380651 Free PMC article.
-
Divalent Metal Uptake and the Role of ZIP8 in Host Defense Against Pathogens.Front Cell Dev Biol. 2022 Jun 27;10:924820. doi: 10.3389/fcell.2022.924820. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35832795 Free PMC article. Review.
-
Alcohol-associated intestinal dysbiosis impairs pulmonary host defense against Klebsiella pneumoniae.PLoS Pathog. 2017 Jun 12;13(6):e1006426. doi: 10.1371/journal.ppat.1006426. eCollection 2017 Jun. PLoS Pathog. 2017. PMID: 28604843 Free PMC article.
-
[Gut microbiota and immune crosstalk in metabolic disease].Biol Aujourdhui. 2017;211(1):1-18. doi: 10.1051/jbio/2017008. Epub 2017 Jul 6. Biol Aujourdhui. 2017. PMID: 28682223 Review. French.
Cited by
-
Human Alcohol-Microbiota Mice have Increased Susceptibility to Bacterial Pneumonia.Cells. 2023 Sep 13;12(18):2267. doi: 10.3390/cells12182267. Cells. 2023. PMID: 37759490 Free PMC article.
References
-
- File T.M., Jr., Low D.E., Eckburg P.B., Talbot G.H., Friedland H.D., Lee J., Llorens L., Critchley I., Thye D. Integrated analysis of FOCUS 1 and FOCUS 2: Randomized, doubled-blinded, multicenter phase 3 trials of the efficacy and safety of ceftaroline fosamil versus ceftriaxone in patients with community-acquired pneumonia. Clin. Infect. Dis. 2010;51:1395–1405. doi: 10.1086/657313. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

