Genetic Diversity among Rose Rosette Virus Isolates: A Roadmap towards Studies of Gene Function and Pathogenicity
- PMID: 37242377
- PMCID: PMC10221089
- DOI: 10.3390/pathogens12050707
Genetic Diversity among Rose Rosette Virus Isolates: A Roadmap towards Studies of Gene Function and Pathogenicity
Abstract
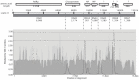
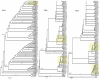
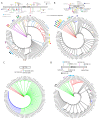
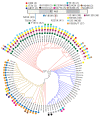
The phylogenetic relationships of ninety-five rose rosette virus (RRV) isolates with full-length genomic sequences were analyzed. These isolates were recovered mostly from commercial roses that are vegetatively propagated rather than grown from seed. First, the genome segments were concatenated, and the maximum likelihood (ML) tree shows that the branches arrange independent of their geographic origination. There were six major groups of isolates, with 54 isolates in group 6 and distributed in two subgroups. An analysis of nucleotide diversity across the concatenated isolates showed lower genetic differences among RNAs encoding the core proteins required for encapsidation than the latter genome segments. Recombination breakpoints were identified near the junctions of several genome segments, suggesting that the genetic exchange of segments contributes to differences among isolates. The ML analysis of individual RNA segments revealed different relationship patterns among isolates, which supports the notion of genome reassortment. We tracked the branch positions of two newly sequenced isolates to highlight how genome segments relate to segments of other isolates. RNA6 has an interesting pattern of single-nucleotide mutations that appear to influence amino acid changes in the protein products derived from ORF6a and ORF6b. The P6a proteins were typically 61 residues, although three isolates encoded P6a proteins truncated to 29 residues, and four proteins extended 76-94 residues. Homologous P5 and P7 proteins appear to be evolving independently. These results suggest greater diversity among RRV isolates than previously recognized.
Keywords: emaravirus; negative-strand RNA virus; plant bunyavirus; rose; rose rosette virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

