Trial of a Novel Oral Cannabinoid Formulation in Patients with Hypertension: A Double-Blind, Placebo-Controlled Pharmacogenetic Study
- PMID: 37242428
- PMCID: PMC10223705
- DOI: 10.3390/ph16050645
Trial of a Novel Oral Cannabinoid Formulation in Patients with Hypertension: A Double-Blind, Placebo-Controlled Pharmacogenetic Study
Abstract
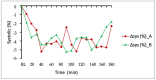
Cannabidiol (CBD) is a non-psychoactive cannabinoid, and available evidence suggests potential efficacy in the treatment of many disorders. DehydraTECH™2.0 CBD is a patented capsule formulation that improves the bioabsorption of CBD. We sought to compare the effects of CBD and DehydraTECH™2.0 CBD based on polymorphisms in CYP P450 genes and investigate the effects of a single CBD dose on blood pressure. In a randomized and double-blinded order, 12 females and 12 males with reported hypertension were given either placebo capsules or DehydraTECH™2.0 CBD (300 mg of CBD, each). Blood pressure and heart rate were measured during 3 h, and blood and urine samples were collected. In the first 20 min following the dose, there was a greater reduction in diastolic blood pressure (p = 0.025) and mean arterial pressure MAP (p = 0.056) with DehydraTECH™2.0 CBD, which was probably due to its greater CBD bioavailability. In the CYP2C9*2*3 enzyme, subjects with the poor metabolizer (PM) phenotype had higher plasma CBD concentrations. Both CYP2C19*2 (p = 0.037) and CYP2C19*17 (p = 0.022) were negatively associated with urinary CBD levels (beta = -0.489 for CYP2C19*2 and beta = -0.494 for CYP2C19*17). Further research is required to establish the impact of CYP P450 enzymes and the identification of metabolizer phenotype for the optimization of CBD formulations.
Keywords: CYP P450 genes; GC-MS analysis; SNP genotyping; blood pressure; cannabidiol.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures






References
-
- Gray R.A., Heal D.J., Maguire D.R., Gerak L.R., Javors M.A., Smith S., France C.P. Preclinical Assessment of the Abuse Potential of Purified Botanical Cannabidiol: Self-Administration, Drug Discrimination, and Physical Dependence. J. Pharmacol. Exp. Ther. 2022;382:54–65. doi: 10.1124/jpet.121.000988. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

