Assessment of Aptamer as a Potential Drug Targeted Delivery for Retinal Angiogenesis Inhibition
- PMID: 37242534
- PMCID: PMC10221175
- DOI: 10.3390/ph16050751
Assessment of Aptamer as a Potential Drug Targeted Delivery for Retinal Angiogenesis Inhibition
Abstract
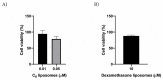
AT11-L0 is an aptamer derivative of AS1411 composed of G-rich sequences that can adopt a G-quadruplex (G4) structure and target nucleolin (NCL), a protein that acts as a co-receptor for several growth factors. Hence, this study aimed to characterize the AT11-L0 G4 structure and its interaction with several ligands for NCL targeting and to evaluate their capacity to inhibit angiogenesis using an in vitro model. The AT11-L0 aptamer was then used to functionalize drug-associated liposomes to increase the bioavailability of the aptamer-based drug in the formulation. Biophysical studies, such as nuclear magnetic resonance, circular dichroism, and fluorescence titrations, were performed to characterize the liposomes functionalized with the AT11-L0 aptamer. Finally, these liposome formulations with the encapsulated drugs were tested on the human umbilical vein endothelial cell (HUVEC) model to assess their antiangiogenic capacity. The results showed that the AT11-L0 aptamer-ligand complexes are highly stable, presenting melting temperatures from 45 °C to 60 °C, allowing for efficient targeting of NCL with a KD in the order of nM. The aptamer-functionalized liposomes loaded with ligands C8 and dexamethasone did not show cytotoxic effects in HUVEC cells compared with the free ligands and AT11-L0, as assessed by cell viability assays. AT11-L0 aptamer-functionalized liposomes encapsulating C8 and dexamethasone did not present a significant reduction in the angiogenic process when compared with the free ligands. In addition, AT11-L0 did not show anti-angiogenic effects at the concentrations tested. However, C8 shows potential as an angiogenesis inhibitor, which should be further developed and optimized in future experiments.
Keywords: G-quadruplex aptamers; angiogenesis; nanosystems; nucleolin; retinal diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Sharma A., Parachuri N., Kumar N., Bandello F., Kuppermann B.D., Loewenstein A., Regillo C.D., Chakravarthy U. Terms Non-exudative and Non-neovascular: Awaiting Entry at the Doors of AMD Reclassification. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021;259:1381–1383. doi: 10.1007/S00417-021-05164-6. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

