Triple-Therapy of Peritoneal Metastasis-Partial-Dehydration under Hyperthermic Condition Combined with Chemotherapy: The First Preliminary In-Vitro Results
- PMID: 37242546
- PMCID: PMC10220674
- DOI: 10.3390/ph16050763
Triple-Therapy of Peritoneal Metastasis-Partial-Dehydration under Hyperthermic Condition Combined with Chemotherapy: The First Preliminary In-Vitro Results
Abstract
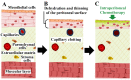
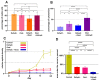
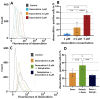
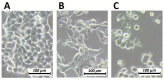
A newly introduced combination of intraperitoneal dehydration and hyperthermia has recently been shown to be feasible and cytotoxic for colon cancer cells in vivo. For the first time, our study now aims to evaluate dehydration under hyperthermic conditions combined with chemotherapy for potential use in the clinical setting. In this study, in vitro colon cancer cells (HT-29) were subjected to single or several cycles of partial dehydration under hyperthermic conditions (45 °C), followed by chemotherapy (triple exposure) with oxaliplatin or doxorubicin in various configurations. The viability, cytotoxicity, and proliferation of cells after the proposed protocols were assessed. Intracellular doxorubicin uptake was measured via flow cytometry. After one cycle of triple exposure, the viability of HT-29 cells was significantly reduced versus the untreated control (65.11 ± 5%, p < 0.0001) and versus only chemotherapy (61.2 ± 7%, p < 0.0001). An increased chemotherapeutic inflow into the cells after triple exposure was detected (53.4 ± 11%) when compared to cells treated with chemotherapy alone (34.23 ± 10%) (p < 0.001). Partial dehydration in a hyperthermic condition combined with chemotherapy increases the overall cytotoxicity of colon cancer cells significantly compared to chemotherapy alone. This could possibly be related to enhanced intracellular uptake of chemotherapeutic agents after partial dehydration. Further studies are required for the further evaluation of this new concept.
Keywords: colorectal cancer; dehydration; doxorubicin; hyperthermia; intraperitoneal chemotherapy; oxaliplatin; peritoneal metastasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Sadeghi B., Arvieux C., Glehen O., Beaujard A.C., Rivoire M., Baulieux J., Fontaumard E., Brachet A., Caillot J.L., Faure J.L., et al. Peritoneal carcinomatosis from non-gynecologic malignancies: Results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000;88:358–363. doi: 10.1002/(SICI)1097-0142(20000115)88:2<358::AID-CNCR16>3.0.CO;2-O. - DOI - PubMed
-
- Anwar A., Kasi A. StatPearls. StatPearls Publishing; Tampa, FL, USA: 2022. Peritoneal Cancer. - PubMed
LinkOut - more resources
Full Text Sources

