Evidence of Strong Guest-Host Interactions in Simvastatin Loaded in Mesoporous Silica MCM-41
- PMID: 37242562
- PMCID: PMC10222570
- DOI: 10.3390/pharmaceutics15051320
Evidence of Strong Guest-Host Interactions in Simvastatin Loaded in Mesoporous Silica MCM-41
Abstract
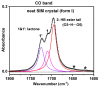
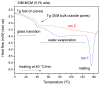
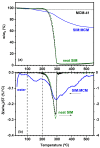
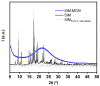
A rational design of drug delivery systems requires in-depth knowledge not only of the drug itself, in terms of physical state and molecular mobility, but also of how it is distributed among a carrier and its interactions with the host matrix. In this context, this work reports the behavior of simvastatin (SIM) loaded in mesoporous silica MCM-41 matrix (average pore diameter ~3.5 nm) accessed by a set of experimental techniques, evidencing that it exists in an amorphous state (X-ray diffraction, ssNMR, ATR-FTIR, and DSC). The most significant fraction of SIM molecules corresponds to a high thermal resistant population, as shown by thermogravimetry, and which interacts strongly with the MCM silanol groups, as revealed by ATR-FTIR analysis. These findings are supported by Molecular Dynamics (MD) simulations predicting that SIM molecules anchor to the inner pore wall through multiple hydrogen bonds. This anchored molecular fraction lacks a calorimetric and dielectric signature corresponding to a dynamically rigid population. Furthermore, differential scanning calorimetry showed a weak glass transition that is shifted to lower temperatures compared to bulk amorphous SIM. This accelerated molecular population is coherent with an in-pore fraction of molecules distinct from bulklike SIM, as highlighted by MD simulations. MCM-41 loading proved to be a suitable strategy for a long-term stabilization (at least three years) of simvastatin in the amorphous form, whose unanchored population releases at a much higher rate compared to the crystalline drug dissolution. Oppositely, the surface-attached molecules are kept entrapped inside pores even after long-term release assays.
Keywords: amorphous state; drug delivery development; drug release; drug-carrier multiple interactions; molecular mobility; simvastatin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




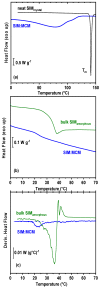


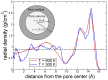



References
-
- Kasim N.A., Whitehouse M., Ramachandran C., Bermejo M., Lennernäs H., Hussain A.S., Junginger H.E., Stavchansky S.A., Midha K.K., Shah V.P., et al. Molecular Properties of WHO Essential Drugs and Provisional Biopharmaceutical Classification. Mol. Pharm. 2004;1:85–96. doi: 10.1021/mp034006h. - DOI - PubMed
-
- DrugStats Database ClinCalc DrugStats Database. [(accessed on 26 March 2023)]. Available online: https://clincalc.com/DrugStats/
-
- Ellison D.K., Moore W.D., Petts C.R. Simvastatin. In: Brittain H.G., editor. Analytical Profiles of Drug Substances and Excipients. Elsevier; Amsterdam, The Netherlands: 1993. pp. 359–388.
-
- Shitara Y., Sugiyama Y. Pharmacokinetic and Pharmacodynamic Alterations of 3-Hydroxy-3-Methylglutaryl Coenzyme A (HMG-CoA) Reductase Inhibitors: Drug–Drug Interactions and Interindividual Differences in Transporter and Metabolic Enzyme Functions. Pharmacol. Ther. 2006;112:71–105. doi: 10.1016/j.pharmthera.2006.03.003. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

