From Batch to the Semi-Continuous Flow Hydrogenation of p NB, p NZ-Protected Meropenem
- PMID: 37242564
- PMCID: PMC10224265
- DOI: 10.3390/pharmaceutics15051322
From Batch to the Semi-Continuous Flow Hydrogenation of p NB, p NZ-Protected Meropenem
Abstract
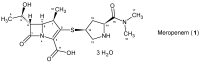
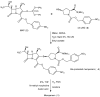
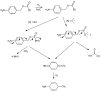
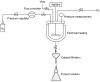
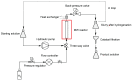
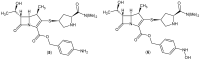
Meropenem is currently the most common carbapenem in clinical applications. Industrially, the final synthetic step is characterized by a heterogeneous catalytic hydrogenation in batch mode with hydrogen and Pd/C. The required high-quality standard is very difficult to meet and specific conditions are required to remove both protecting groups [i.e., p-nitrobenzyl (pNB) and p-nitrobenzyloxycarbonyl (pNZ)] simultaneously. The three-phase gas-liquid-solid system makes this step difficult and unsafe. The introduction of new technologies for small-molecule synthesis in recent years has opened up new landscapes in process chemistry. In this context, we have investigated meropenem hydrogenolysis using microwave (MW)-assisted flow chemistry for use as a new technology with industrial prospects. The reaction parameters (catalyst amount, T, P, residence time, flow rate) in the move from the batch process to semi-continuous flow were investigated under mild conditions to determine their influence on the reaction rate. The optimization of the residence time (840 s) and the number of cycles (4) allowed us to develop a novel protocol that halves the reaction time compared to batch production (14 min vs. 30 min) while maintaining the same product quality. The increase in productivity using this semi-continuous flow technique compensates for the slightly lower yield (70% vs. 74%) obtained in batch mode.
Keywords: drug synthesis; flow chemistry; heterogeneous catalysis; hydrogenation; meropenem; microwave-assisted; miniaturization; semi-continuous synthesis; sustainability.
Conflict of interest statement
All authors declare no conflict of interest.
Figures