Recent Advances of Fe(III)/Fe(II)-MPNs in Biomedical Applications
- PMID: 37242566
- PMCID: PMC10223096
- DOI: 10.3390/pharmaceutics15051323
Recent Advances of Fe(III)/Fe(II)-MPNs in Biomedical Applications
Abstract
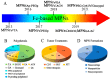
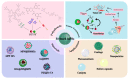
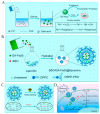
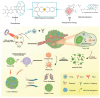
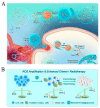
Metal-phenolic networks (MPNs) are a new type of nanomaterial self-assembled by metal ions and polyphenols that have been developed rapidly in recent decades. They have been widely investigated, in the biomedical field, for their environmental friendliness, high quality, good bio-adhesiveness, and bio-compatibility, playing a crucial role in tumor treatment. As the most common subclass of the MPNs family, Fe-based MPNs are most frequently used in chemodynamic therapy (CDT) and phototherapy (PTT), where they are often used as nanocoatings to encapsulate drugs, as well as good Fenton reagents and photosensitizers to improve tumor therapeutic efficiency substantially. In this review, strategies for preparing various types of Fe-based MPNs are first summarized. We highlight the advantages of Fe-based MPNs under the different species of polyphenol ligands for their application in tumor treatments. Finally, some current problems and challenges of Fe-based MPNs, along with a future perspective on biomedical applications, are discussed.
Keywords: CDT; Fe-based MPN; Fenton reaction; PTT; tumor treatment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



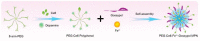
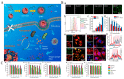


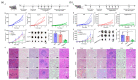



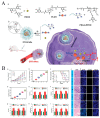

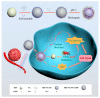


References
-
- Yang K., Zhao S.J., Li B.L., Wang B.H., Lan M.H., Song X.Z. Low temperature photothermal therapy: Advances and perspectives. Coordin. Chem. Rev. 2022;454:214330. doi: 10.1016/j.ccr.2021.214330. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

