Urtica dioica Agglutinin Prevents Rabies Virus Infection in a Muscle Explant Model
- PMID: 37242595
- PMCID: PMC10221701
- DOI: 10.3390/pharmaceutics15051353
Urtica dioica Agglutinin Prevents Rabies Virus Infection in a Muscle Explant Model
Abstract
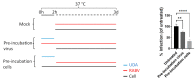
Infection with the rabies virus (RABV) results in a 100% lethal neurological disease once symptoms develop. Post-exposure prophylaxis (PEP) consists of a combination of vaccination and anti-rabies immunoglobulins (RIGs); it is 100% effective if administered early after exposure. Because of its limited availability, alternatives for RIGs are needed. To that end, we evaluated a panel of 33 different lectins for their effect on RABV infection in cell culture. Several lectins, with either mannose or GlcNAc specificity, elicited anti-RABV activity, of which the GlcNAc-specific Urtica dioica agglutinin (UDA) was selected for further studies. UDA was found to prevent the entry of the virus into the host cell. To further assess the potential of UDA, a physiologically relevant RABV infection muscle explant model was developed. Strips of dissected swine skeletal muscle that were kept in a culture medium could be productively infected with the RABV. When the infection of the muscle strips was carried out in the presence of UDA, RABV replication was completely prevented. Thus, we developed a physiologically relevant RABV muscle infection model. UDA (i) may serve as a reference for further studies and (ii) holds promise as a cheap and simple-to-produce alternative for RIGs in PEP.
Keywords: antiviral; lectin; muscle explant; rabies virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Studies on the binding of Urtica dioica agglutinin (UDA) and other lectins in an in vitro epidermal growth factor receptor test.Phytomedicine. 1995 Apr;1(4):287-90. doi: 10.1016/S0944-7113(11)80004-8. Phytomedicine. 1995. PMID: 23196016
-
Identification and Characterization of a Small-Molecule Rabies Virus Entry Inhibitor.J Virol. 2020 Jun 16;94(13):e00321-20. doi: 10.1128/JVI.00321-20. Print 2020 Jun 16. J Virol. 2020. PMID: 32321812 Free PMC article.
-
Effects of Urtica dioica agglutinin on glycotargeting of the vasculature: an in ovo study on chicken embryo.Vet Res Forum. 2022 Sep;13(3):379-385. doi: 10.30466/vrf.2020.136565.3057. Epub 2022 Sep 15. Vet Res Forum. 2022. PMID: 36320306 Free PMC article.
-
Research Advances on the Interactions between Rabies Virus Structural Proteins and Host Target Cells: Accrued Knowledge from the Application of Reverse Genetics Systems.Viruses. 2021 Nov 16;13(11):2288. doi: 10.3390/v13112288. Viruses. 2021. PMID: 34835093 Free PMC article. Review.
-
Monoclonal antibodies against rabies: current uses in prophylaxis and in therapy.Curr Opin Virol. 2022 Apr;53:101204. doi: 10.1016/j.coviro.2022.101204. Epub 2022 Feb 10. Curr Opin Virol. 2022. PMID: 35151116 Review.
Cited by
-
A Robust Phenotypic High-Throughput Antiviral Assay for the Discovery of Rabies Virus Inhibitors.Viruses. 2023 Nov 23;15(12):2292. doi: 10.3390/v15122292. Viruses. 2023. PMID: 38140533 Free PMC article.
-
Urtica dioica: Anticancer Properties and Other Systemic Health Benefits from In Vitro to Clinical Trials.Int J Mol Sci. 2024 Jul 8;25(13):7501. doi: 10.3390/ijms25137501. Int J Mol Sci. 2024. PMID: 39000608 Free PMC article. Review.
-
Carnosic Acid Inhibits Herpes Simplex Virus Replication by Suppressing Cellular ATP Synthesis.Int J Mol Sci. 2024 May 3;25(9):4983. doi: 10.3390/ijms25094983. Int J Mol Sci. 2024. PMID: 38732202 Free PMC article.
References
-
- WHO . WHO Guide for Rabies Pre and Post-Exposure Prophylaxis in Humans. World Health Organization; Geneva, Switzerland: 2014. pp. 1–21.
-
- Sreenivasan N., Li A., Shiferaw M., Tran C.H., Wallace R., Blanton J., Knopf L., Abela-Ridder B., Hyde T., Siddiqi U.R., et al. Overview of rabies post-exposure prophylaxis access, procurement and distribution in selected countries in Asia and Africa, 2017–2018. Vaccine. 2019;37:A6–A13. doi: 10.1016/j.vaccine.2019.04.024. - DOI - PMC - PubMed
-
- Wang H., Zhao W., Zhang S.F., Miao F.M., Cao Y., Chen C., Li Y.F., Gao J., Lv R.Y., Zhang S.X., et al. Efficacy of ormutivimab, a novel recombinant human anti-rabies monoclonal antibody, in post-exposure prophylaxis animal models. Travel Med. Infect. Dis. 2022;46:102267. doi: 10.1016/j.tmaid.2022.102267. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

