A Novel Approach for the Treatment of Aerobic Vaginitis: Azithromycin Liposomes-in-Chitosan Hydrogel
- PMID: 37242598
- PMCID: PMC10221335
- DOI: 10.3390/pharmaceutics15051356
A Novel Approach for the Treatment of Aerobic Vaginitis: Azithromycin Liposomes-in-Chitosan Hydrogel
Abstract
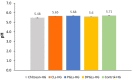
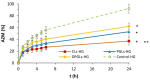
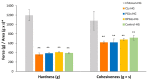
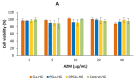
Biocompatible mucoadhesive formulations that enable a sustained drug delivery at the site of action, while exhibiting inherent antimicrobial activity, are of great importance for improved local therapy of vaginal infections. The aim of this research was to prepare and evaluate the potential of the several types of azithromycin (AZM)-liposomes (180-250 nm) incorporated into chitosan hydrogel (AZM-liposomal hydrogels) for the treatment of aerobic vaginitis. AZM-liposomal hydrogels were characterized for in vitro release, and rheological, texture, and mucoadhesive properties under conditions simulating the vaginal site of application. The role of chitosan as a hydrogel-forming polymer with intrinsic antimicrobial properties was explored against several bacterial strains typical for aerobic vaginitis as well as its potential effect on the anti-staphylococcal activity of AZM-liposomes. Chitosan hydrogel prolonged the release of the liposomal drug and exhibited inherent antimicrobial activity. Additionally, it boosted the antibacterial effect of all tested AZM-liposomes. All AZM-liposomal hydrogels were biocompatible with the HeLa cells and demonstrated mechanical properties suitable for vaginal application, thus confirming their potential for enhanced local therapy of aerobic vaginitis.
Keywords: aerobic vaginitis; antimicrobial activity; azithromycin; chitosan; hydrogel; liposomes; vaginal drug delivery; vaginal infections.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

