Isolation of Secondary Metabolites from Achillea grandifolia Friv. (Asteraceae) and Main Compounds' Effects on a Glioblastoma Cellular Model
- PMID: 37242625
- PMCID: PMC10224520
- DOI: 10.3390/pharmaceutics15051383
Isolation of Secondary Metabolites from Achillea grandifolia Friv. (Asteraceae) and Main Compounds' Effects on a Glioblastoma Cellular Model
Abstract
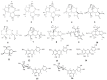
This study aims at the isolation and structural determination of the secondary metabolites of the herbaceous perennial plant Achillea grandifolia Friv. (Asteraceae). The examination of the non-volatile content of the leaves and flowers of A. grandifolia afforded the isolation of sixteen secondary metabolites. On the basis of NMR spectra, the identified compounds included ten sesquiterpene lactones; three guaianolides-rupicolin A (1), rupicolin B (2), and (4S,6aS,9R,9aS,9bS)-4,6a,9-trihydroxy-9-methyl-3,6-dimethylene-3a,4,5,6,6a,9,9a,9b-octahydro-3H-azuleno [4,5-b]furan-2-one (3); two eudesmanolides-artecalin (4) and ridentin B (5); two sesquiterpene methyl esters-(1S,2S,4αR,5R,8R,8αS)-decahydro-1,5,8-trihydroxy-4α,8-dimethyl-methylene-2-naphthaleneacetic acid methylester (6) and 1β, 3β, 6α-trihydroxycostic acid methyl ester (7); three secoguaianolides-acrifolide (8), arteludovicinolide A (9), and lingustolide A (10); and an iridoid-loliolide (11). Moreover, five known flavonoids, i.e., apigenin, luteolin, eupatolitin, apigenin 7-O-glucoside, and luteolin 7-O-glucoside (12-16) were also purified from the aerial parts of the plant material. We also investigated the effect of rupicolin A (1) and B (2) (main compounds) on U87MG and T98G glioblastoma cell lines. An MTT assay was performed to define cytotoxic effects and to calculate the IC50, while flow cytometry was employed to analyze the cell cycle. The IC50 values of reduced viability during the 48 h treatment for compound (1) and (2) were 38 μM and 64 μM for the U87MG cells and 15 μM and 26 μM for the T98G cells, respectively. Both rupicolin A and B induced a G2/M cell cycle arrest.
Keywords: cell lines; flavonoids; glioblastoma; rupicolin A; rupicolin B; sesquiterpene lactones.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Radulović N., Zlatković B., Palić R., Stojanović G. Chemotaxonomic significance of the Balkan Achillea volatiles. Nat. Prod. Commun. 2007;2:453–474. doi: 10.1177/1934578X0700200417. - DOI
-
- Stanković N., Mihajilov-Krstev T., Zlatković B., Stankov-Jovanović V., Mitić V., Jović J., Čomić L., Kocić B., Bernstein N. Antibacterial and antioxidant activity of traditional medicinal plants from the Balkan peninsula. NJAS Wagen. J. Life Sc. 2016;78:21–28. doi: 10.1016/j.njas.2015.12.006. - DOI
-
- Choucry M.A. Chemical composition and anticancer activity of Achillea fragrantissima (Forssk.) Sch. Bip. (Asteraceae) essential oil from Egypt. J. Pharmacogn. Phytother. 2017;9:1–5. doi: 10.5897/JPP2015.0399. - DOI
LinkOut - more resources
Full Text Sources

