Emerging Roles of Mesenchymal Stem/Stromal-Cell-Derived Extracellular Vesicles in Cancer Therapy
- PMID: 37242693
- PMCID: PMC10221697
- DOI: 10.3390/pharmaceutics15051453
Emerging Roles of Mesenchymal Stem/Stromal-Cell-Derived Extracellular Vesicles in Cancer Therapy
Abstract
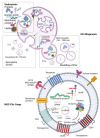
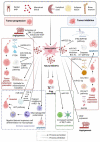
Despite the tremendous efforts of many researchers and clinicians, cancer remains the second leading cause of mortality worldwide. Mesenchymal stem/stromal cells (MSCs) are multipotent cells residing in numerous human tissues and presenting unique biological properties, such as low immunogenicity, powerful immunomodulatory and immunosuppressive capabilities, and, in particular, homing abilities. Therapeutic functions of MSCs are mediated mostly by the paracrine effect of released functional molecules and other variable components, and among them the MSC-derived extracellular vesicles (MSC-EVs) seem to be one of the central mediators of the therapeutic functions of MSCs. MSC-EVs are membrane structures secreted by the MSCs, rich in specific proteins, lipids, and nucleic acids. Amongst these, microRNAs have achieved the most attention currently. Unmodified MSC-EVs can promote or inhibit tumor growth, while modified MSC-EVs are involved in the suppression of cancer progression via the delivery of therapeutic molecules, including miRNAs, specific siRNAs, or suicide RNAs, as well as chemotherapeutic drugs. Here, we present an overview of the characteristics of the MSCs-EVs and describe the current methods for their isolation and analysis, the content of their cargo, and modalities for the modification of MSC-EVs in order for them to be used as drug delivery vehicles. Finally, we describe different roles of MSC-EVs in the tumor microenvironment and summarize current advances of MCS-EVs in cancer research and therapy. MSC-EVs are expected to be a novel and promising cell-free therapeutic drug delivery vehicle for the treatment of cancer.
Keywords: cancer therapy; cell-free therapy; drug delivery vehicle; extracellular vesicles; mesenchymal cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Mesenchymal stromal cell-derived extracellular vesicles: regenerative and immunomodulatory effects and potential applications in sepsis.Cell Tissue Res. 2018 Oct;374(1):1-15. doi: 10.1007/s00441-018-2871-5. Epub 2018 Jun 28. Cell Tissue Res. 2018. PMID: 29955951 Review.
-
Mesenchymal Stromal Cell-Derived Extracellular Vesicles Attenuate Dendritic Cell Maturation and Function.Front Immunol. 2018 Nov 9;9:2538. doi: 10.3389/fimmu.2018.02538. eCollection 2018. Front Immunol. 2018. PMID: 30473695 Free PMC article.
-
Human multipotent mesenchymal stromal cells cytokine priming promotes RAB27B-regulated secretion of small extracellular vesicles with immunomodulatory cargo.Stem Cell Res Ther. 2020 Dec 14;11(1):539. doi: 10.1186/s13287-020-02050-6. Stem Cell Res Ther. 2020. PMID: 33317598 Free PMC article.
-
Unveiling the multifaceted roles of microRNAs in extracellular vesicles derived from mesenchymal stem cells: implications in tumor progression and therapeutic interventions.Front Pharmacol. 2024 Aug 5;15:1438177. doi: 10.3389/fphar.2024.1438177. eCollection 2024. Front Pharmacol. 2024. PMID: 39161894 Free PMC article. Review.
-
Therapeutic potential of mesenchymal stem cell-derived extracellular vesicles as novel cell-free therapy for treatment of autoimmune disorders.Exp Mol Pathol. 2021 Feb;118:104566. doi: 10.1016/j.yexmp.2020.104566. Epub 2020 Nov 6. Exp Mol Pathol. 2021. PMID: 33160961 Review.
Cited by
-
Exosome-based miRNA delivery: Transforming cancer treatment with mesenchymal stem cells.Regen Ther. 2025 Feb 13;28:558-572. doi: 10.1016/j.reth.2025.01.019. eCollection 2025 Mar. Regen Ther. 2025. PMID: 40034540 Free PMC article. Review.
-
Noncoding RNAs in Hepatocellular Carcinoma: Potential Applications in Combined Therapeutic Strategies and Promising Candidates of Treatment Response.Cancers (Basel). 2024 Feb 13;16(4):766. doi: 10.3390/cancers16040766. Cancers (Basel). 2024. PMID: 38398157 Free PMC article. Review.
-
Gemcitabine and Flurbiprofen Enhance Cytotoxic Effects on Cancer Cell Lines Mediated by Mesenchymal Stem Cells.Int J Mol Sci. 2025 Jun 27;26(13):6212. doi: 10.3390/ijms26136212. Int J Mol Sci. 2025. PMID: 40649990 Free PMC article.
-
Mesenchymal stem cells and mesenchymal stem cell-derived exosomes: a promising strategy for treating retinal degenerative diseases.Mol Med. 2025 Feb 21;31(1):75. doi: 10.1186/s10020-025-01120-w. Mol Med. 2025. PMID: 39984849 Free PMC article. Review.
-
Tumor-derived extracellular vesicles regulate macrophage polarization: role and therapeutic perspectives.Front Immunol. 2024 Apr 16;15:1346587. doi: 10.3389/fimmu.2024.1346587. eCollection 2024. Front Immunol. 2024. PMID: 38690261 Free PMC article.
References
-
- Caplan H., Olson S.D., Kumar A., George M., Prabhakara K.S., Wenzel P., Bedi S., Toledano-Furman N.E., Triolo F., Kamhieh-Milz J., et al. Mesenchymal Stromal Cell Therapeutic Delivery: Translational Challenges to Clinical Application. Front. Immunol. 2019;10:1645. doi: 10.3389/fimmu.2019.01645. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

