Cationic Microbubbles for Non-Selective Binding of Cavitation Nuclei to Bacterial Biofilms
- PMID: 37242736
- PMCID: PMC10221258
- DOI: 10.3390/pharmaceutics15051495
Cationic Microbubbles for Non-Selective Binding of Cavitation Nuclei to Bacterial Biofilms
Abstract
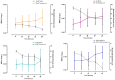
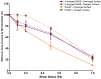
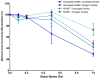
The presence of multi-drug resistant biofilms in chronic, persistent infections is a major barrier to successful clinical outcomes of therapy. The production of an extracellular matrix is a characteristic of the biofilm phenotype, intrinsically linked to antimicrobial tolerance. The heterogeneity of the extracellular matrix makes it highly dynamic, with substantial differences in composition between biofilms, even in the same species. This variability poses a major challenge in targeting drug delivery systems to biofilms, as there are few elements both suitably conserved and widely expressed across multiple species. However, the presence of extracellular DNA within the extracellular matrix is ubiquitous across species, which alongside bacterial cell components, gives the biofilm its net negative charge. This research aims to develop a means of targeting biofilms to enhance drug delivery by developing a cationic gas-filled microbubble that non-selectively targets the negatively charged biofilm. Cationic and uncharged microbubbles loaded with different gases were formulated and tested to determine their stability, ability to bind to negatively charged artificial substrates, binding strength, and, subsequently, their ability to adhere to biofilms. It was shown that compared to their uncharged counterparts, cationic microbubbles facilitated a significant increase in the number of microbubbles that could both bind and sustain their interaction with biofilms. This work is the first to demonstrate the utility of charged microbubbles for the non-selective targeting of bacterial biofilms, which could be used to significantly enhance stimuli-mediated drug delivery to the bacterial biofilm.
Keywords: Biofilm; cationic microbubble; cavitation nuclei; drug delivery; microbubble; microbubble targeting; ultrasound drug delivery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- O’Neil J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. 2014. [(accessed on 11 May 2023)]. Available online: https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Ta....
Grants and funding
LinkOut - more resources
Full Text Sources

