Differential Expression of ABC Transporter Genes in Brain Vessels vs. Peripheral Tissues and Vessels from Human, Mouse and Rat
- PMID: 37242805
- PMCID: PMC10221359
- DOI: 10.3390/pharmaceutics15051563
Differential Expression of ABC Transporter Genes in Brain Vessels vs. Peripheral Tissues and Vessels from Human, Mouse and Rat
Abstract
Background: ATP-binding cassette (ABC) transporters comprise a superfamily of genes encoding membrane proteins with nucleotide-binding domains (NBD). These transporters, including drug efflux across the blood-brain barrier (BBB), carry a variety of substrates through plasma membranes against substrate gradients, fueled by hydrolyzing ATP. The expression patterns/enrichment of ABC transporter genes in brain microvessels compared to peripheral vessels and tissues are largely uncharacterized.
Methods: In this study, the expression patterns of ABC transporter genes in brain microvessels, peripheral tissues (lung, liver and spleen) and lung vessels were investigated using RNA-seq and WesTM analyses in three species: human, mouse and rat.
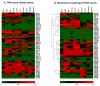
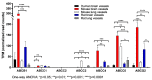
Results: The study demonstrated that ABC drug efflux transporter genes (including ABCB1, ABCG2, ABCC4 and ABCC5) were highly expressed in isolated brain microvessels in all three species studied; the expression of ABCB1, ABCG2, ABCC1, ABCC4 and ABCC5 was generally higher in rodent brain microvessels compared to those of humans. In contrast, ABCC2 and ABCC3 expression was low in brain microvessels, but high in rodent liver and lung vessels. Overall, most ABC transporters (with the exception of drug efflux transporters) were enriched in peripheral tissues compared to brain microvessels in humans, while in rodent species, additional ABC transporters were found to be enriched in brain microvessels.
Conclusions: This study furthers the understanding of species similarities and differences in the expression patterns of ABC transporter genes; this is important for translational studies in drug development. In particular, CNS drug delivery and toxicity may vary among species depending on their unique profiles of ABC transporter expression in brain microvessels and BBB.
Keywords: ABC transporters; RNA-seq; WesTM analysis; across species; brain microvessels; gene expression patterns; peripheral tissues and vessels.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Comparative gene expression profiles of ABC transporters in brain microvessel endothelial cells and brain in five species including human.Pharmacol Res. 2009 Jun;59(6):404-13. doi: 10.1016/j.phrs.2009.02.007. Epub 2009 Feb 24. Pharmacol Res. 2009. PMID: 19429473
-
ABC transporters, cytochromes P450 and their main transcription factors: expression at the human blood-brain barrier.J Neurochem. 2008 Dec;107(6):1518-28. doi: 10.1111/j.1471-4159.2008.05720.x. J Neurochem. 2008. PMID: 19094056
-
Ontogeny of ABC and SLC transporters in the microvessels of developing rat brain.Fundam Clin Pharmacol. 2016 Apr;30(2):107-16. doi: 10.1111/fcp.12175. Epub 2016 Feb 4. Fundam Clin Pharmacol. 2016. PMID: 26662930
-
Genetic polymorphisms of ATP-binding cassette transporters ABCB1 and ABCC2 and their impact on drug disposition.Curr Drug Targets. 2011 May;12(5):631-46. doi: 10.2174/138945011795378487. Curr Drug Targets. 2011. PMID: 21039333 Review.
-
Interplay of drug metabolizing CYP450 enzymes and ABC transporters in the blood-brain barrier.Curr Drug Metab. 2011 Oct;12(8):732-41. doi: 10.2174/138920011798357024. Curr Drug Metab. 2011. PMID: 21623707 Review.
Cited by
-
Interaction of mercury exposure and DNA methylation with sustained attention in children in a novel analysis of epigenetic susceptibility.Environ Epigenet. 2025 Apr 24;11(1):dvaf011. doi: 10.1093/eep/dvaf011. eCollection 2025. Environ Epigenet. 2025. PMID: 40401167 Free PMC article.
-
Blood-Brain Barrier (BBB)-Crossing Strategies for Improved Treatment of CNS Disorders.Handb Exp Pharmacol. 2024;284:213-230. doi: 10.1007/164_2023_689. Handb Exp Pharmacol. 2024. PMID: 37528323
-
Role of ABCC5 in cancer drug resistance and its potential as a therapeutic target.Front Cell Dev Biol. 2024 Nov 5;12:1446418. doi: 10.3389/fcell.2024.1446418. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39563862 Free PMC article. Review.
-
Circadian Rhythms of the Blood-Brain Barrier and Drug Delivery.Circ Res. 2024 Mar 15;134(6):727-747. doi: 10.1161/CIRCRESAHA.123.323521. Epub 2024 Mar 14. Circ Res. 2024. PMID: 38484027 Free PMC article. Review.
-
Circadian influences on central nervous system barriers and the glymphatic system.Front Physiol. 2025 Jun 23;16:1622236. doi: 10.3389/fphys.2025.1622236. eCollection 2025. Front Physiol. 2025. PMID: 40626046 Free PMC article. Review.
References
-
- Zhang W., Bamji-Mirza M., Chang N., Haqqani A., Stanimirovic D.B. Expression and Function of ABC Transporters at the Blood-Brain Barrier. In: Dorovini-Zis K., editor. The Blood-Brain Barrier in Health and Disease. Volume one. CRC press/Taylor & Francis Group; New York, NY, USA: 2015. pp. 172–214.
-
- Dezi M., Fribourg P.F., Di Cicco A., Arnaud O., Marco S., Falson P., Di Pietro A., Lévy D. The multidrug resistance half-transporter ABCG2 is purified as a tetramer upon selective extraction from membranes. Biochim. Biophys. Acta. 2010;1798:2094–2101. doi: 10.1016/j.bbamem.2010.07.034. - DOI - PubMed
-
- Shimabuku A.M., Nishimoto T., Ueda K., Komano T. P-glycoprotein. ATP hydrolysis by the N-terminal nucleotide-binding domain. J. Biol. Chem. 1992;267:4308–4311. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

