Liposome and QS-21 Combined Adjuvant Induces theHumoral and Cellular Responses of Acellular Pertussis Vaccine in a Mice Model
- PMID: 37243018
- PMCID: PMC10223510
- DOI: 10.3390/vaccines11050914
Liposome and QS-21 Combined Adjuvant Induces theHumoral and Cellular Responses of Acellular Pertussis Vaccine in a Mice Model
Abstract
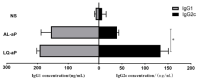
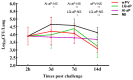
The resurgence of pertussis in vaccinated communities may be related to the reduced long-term immunity induced by acellular pertussis vaccines. Therefore, developing improved pertussis vaccine candidates that could induce strong Th1 or Th17 cellular immunity is an urgent need. The use of new adjuvants may well meet this requirement. In this research, we developed a novel adjuvant candidate by combining liposome and QS-21 adjuvant. Adjuvant activity, protective efficacy, the level of neutralizing antibody against PT, and the resident memory T (TRM) cells in lung tissue after vaccination were studied. We then performed B. pertussis respiratory challenge in mice after they received vaccination with traditional aluminum hydroxide and the novel adjuvant combination. Results showed that the liposome + QS-21 adjuvant group had a rapid antibody and higher antibody (PT, FHA, Fim) level, induced anti-PT neutralizing antibody and recruited more IL-17A-secreting CD4+ TRM cells along with IL-17A-secreting CD8+ TRM cells in mice, which provided robust protection against B. pertussis infection. These results provide a key basis for liposome + QS-21 adjuvant as a promising adjuvant candidate for developing an acellular pertussis vaccine that elicits protective immunity against pertussis.
Keywords: Bordetella pertussis; adjuvant; mouse model; tissue-resident memory T cell; vaccine.
Conflict of interest statement
All authors confirm that there are no conflicts of interest in this work.
Figures




Similar articles
-
Sustained protective immunity against Bordetella pertussis nasal colonization by intranasal immunization with a vaccine-adjuvant combination that induces IL-17-secreting TRM cells.Mucosal Immunol. 2018 Nov;11(6):1763-1776. doi: 10.1038/s41385-018-0080-x. Epub 2018 Aug 20. Mucosal Immunol. 2018. PMID: 30127384
-
A Pertussis Outer Membrane Vesicle-Based Vaccine Induces Lung-Resident Memory CD4 T Cells and Protection Against Bordetella pertussis, Including Pertactin Deficient Strains.Front Cell Infect Microbiol. 2019 Apr 26;9:125. doi: 10.3389/fcimb.2019.00125. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31106160 Free PMC article.
-
Addition of a TLR7 agonist to an acellular pertussis vaccine enhances Th1 and Th17 responses and protective immunity in a mouse model.Vaccine. 2017 Sep 18;35(39):5256-5263. doi: 10.1016/j.vaccine.2017.08.009. Epub 2017 Aug 18. Vaccine. 2017. PMID: 28823618
-
Next-Generation Pertussis Vaccines Based on the Induction of Protective T Cells in the Respiratory Tract.Vaccines (Basel). 2020 Oct 21;8(4):621. doi: 10.3390/vaccines8040621. Vaccines (Basel). 2020. PMID: 33096737 Free PMC article. Review.
-
[Novel vaccines against M. tuberculosis].Kekkaku. 2006 Dec;81(12):745-51. Kekkaku. 2006. PMID: 17240920 Review. Japanese.
Cited by
-
Development of semisynthetic saponin immunostimulants.Med Chem Res. 2024;33(8):1292-1306. doi: 10.1007/s00044-024-03227-x. Epub 2024 May 18. Med Chem Res. 2024. PMID: 39132259 Free PMC article. Review.
References
-
- Jia J.H., Guo Q., Wan C.M. Resurgence and vaccine strategies of pertussis. Zhonghua Er Ke Za Zhi. 2020;58:686–689. - PubMed
-
- Menzies R., Mcintyre P., Beard F. Vaccine preventable diseases and vaccination coverage in Aboriginal and Torres Strait Islander people, Australia, 1999 to 2002. Commun. Dis. Intell. Q. Rep. 2004;28:127–159. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

