Associated Factors and Immune Response to the Hepatitis B Vaccine with a Standard Schedule: A Prospective Study of People with HIV in China
- PMID: 37243025
- PMCID: PMC10224281
- DOI: 10.3390/vaccines11050921
Associated Factors and Immune Response to the Hepatitis B Vaccine with a Standard Schedule: A Prospective Study of People with HIV in China
Abstract
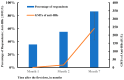
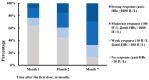
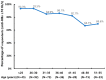
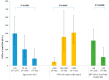
Hepatitis B (HB) vaccination is recommended for people with human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS). We aimed to assess the immune response to the HB vaccine and associated factors using the standard vaccination schedule among people with HIV (PWH) in China. A prospective study was carried out from 2016 to 2020 in Beijing, China. PWH were given three 20 μg doses of recombinant HB vaccine at 0, 1, and 6 months. Blood samples were taken within 4-6 weeks after each dose to evaluate the anti-HBs levels. A total of 312 participants completed vaccination and serologic testing. The seroconversion (anti-HBs ≥ 10 IU/L) rates following the first, second, and third doses of the vaccine were 35.6% (95% CI: 30.3-40.9%), 55.1% (95% CI: 49.6-60.7%), and 86.5% (95% CI: 82.8-90.3%), respectively, and the geometric means of the anti-HBs titers were 0.8 IU/L (95% CI: 0.5-1.6 IU/L), 15.7 IU/L (95% CI: 9.4-26.3 IU/L), and 241.0 IU/L (95% CI: 170.3-341.1 IU/L), respectively. In multivariate analysis, after three doses of vaccination, age, CD4 cell count, and HIV-RNA viral load were significantly associated with strong, moderate, and weak response, respectively. These findings confirm that these personal health conditions are related to the HB response. HB vaccination in PWH using the standard schedule was still highly effective in the context of early treatment initiation, especially among participants aged 30 years and younger.
Keywords: China; HIV/AIDS; associated factors; hepatitis B vaccine; immune response.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Comparative Efficacy of a High-Dose vs Standard-Dose Hepatitis B Revaccination Schedule Among Patients With HIV: A Randomized Clinical Trial.JAMA Netw Open. 2021 Aug 2;4(8):e2120929. doi: 10.1001/jamanetworkopen.2021.20929. JAMA Netw Open. 2021. PMID: 34424307 Free PMC article. Clinical Trial.
-
[Efficacy of vaccination against hepatitis B in adult with HIV infection].Przegl Epidemiol. 2007;61(2):339-47. Przegl Epidemiol. 2007. PMID: 17956052 Clinical Trial. Polish.
-
Response to supplementary vaccination with recombinant or plasma hepatitis B vaccine in healthy non-responding children.Vaccine. 1994 Aug;12(10):899-902. doi: 10.1016/0264-410x(94)90032-9. Vaccine. 1994. PMID: 7975831
-
Immune Response to Hepatitis B Virus Vaccine Among People Living With HIV: A Meta-Analysis.Front Immunol. 2021 Dec 22;12:745541. doi: 10.3389/fimmu.2021.745541. eCollection 2021. Front Immunol. 2021. PMID: 35003061 Free PMC article.
-
Efficacy and Durability of Immune Response After Receipt of Hepatitis A Vaccine in People With Human Immunodeficiency Virus.Open Forum Infect Dis. 2025 Apr 11;12(4):ofaf143. doi: 10.1093/ofid/ofaf143. eCollection 2025 Apr. Open Forum Infect Dis. 2025. PMID: 40225827 Free PMC article. Review.
Cited by
-
Immune response to hepatitis B vaccine among children under 5 years in Africa: a meta-analysis.Trop Med Health. 2024 Apr 1;52(1):28. doi: 10.1186/s41182-024-00594-4. Trop Med Health. 2024. PMID: 38561838 Free PMC article. Review.
-
Immunogenicity and Efficacy of Vaccination in People Living with Human Immunodeficiency Virus.Viruses. 2023 Aug 30;15(9):1844. doi: 10.3390/v15091844. Viruses. 2023. PMID: 37766251 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

